- AI-driven digital health startup Eko raises $20M led by ARTIS Ventures with participation from 3M and Mayo Clinic.
- Funding will be used to accelerate research and development (R&D) and commercialization of Eko’s machine learning platform for cardiac screening/analysis.
- Eko’s cardiac monitor combines digital stethoscope and ECG technology both for in-clinic and at-home monitoring, which enables patients to seamlessly and consistently send cardiac data to their
Read More
ecg
Emory Researchers to Use Wearable ECG Patch to Predict Coronary Artery Disease
· New Emory University study is using VivaLNK’s medical wearable ECG sensors to evaluate autonomic function in predicting coronary artery disease (CAD).· VivaLNK’s ambulatory ECG patch will study patients undergoing coronary angiography in order to measure autonomic function. · The research goal is to better understand the role of the autonomic nervous system in depression and coronary artery disease, and to develop better tools for clinical assessment.An Emory University study is using
Read More
How Tech Giants are Helping Providers Reinvent Patient Care
Healthcare costs are on the rise, and they show no signs of leveling off. According to the latest data available from the Centers for Medicare and Medicaid Services, healthcare spending in the United States reached $3.5 trillion in 2017, or $10,739 per person. Those costs are projected to rise by about five percent year-over-year.
As expenditures continue to skyrocket, healthcare providers are looking to new technologies and care strategies that can help them treat patients more effectively at a
Read More
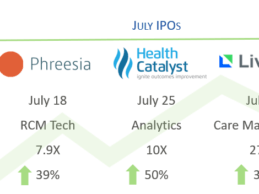
Research: July Digital Health IPOs, M&A Activity, Public Company Performance Summary
Summary of Health IT/digital IPOs, merger & acquisition (M&A) activity, and public company performance during the month of July.
Digital Health IPOs in July
Breaking the digital health IPO drought, there were four initial public offerings (IPOs) during a five-week period from late June to late July, including Livongo, Health Catalyst, Phreesia, and Change Healthcare. The last health IT companies to reach IPO were iRhythym Technologies and Tabula Rasa Healthcare in late 2016 -
Read More
Backed by Mayo Clinic Study, Eko Launches Cardiac Remote Monitoring App, Eko Home
Currently being used in a study by Mayo Clinic, cardiac monitoring app Eko Home enhances outpatient cardiac data collection and demonstrates real world efficacyEko, a Silicon Valley-based digital health company applying machine learning in the fight against heart disease, today announced its cardiac remote monitoring app, Eko Home. The app is a first-of-its-kind service that enables precise remote monitoring of cardiac function using ECG and heart sounds. Eko Home can be used to create drug-data
Read More
ECG and Oncology Resource Consultants Merge to Expand Oncology Consulting Practice
ECG Management Consultants (ECG), a national healthcare consulting firm, announced the expansion of its oncology consulting practice through a merger with Oncology Resource Consultants (ORC). By acquiring the ORC, ECG continues to build on its commitment to excellence in client services and expanding its oncology advisory expertise.Founded in 1990, ORC has provided the oncology community with strategic cancer center and facilities planning, coupled with operations improvement and implementation
Read More
What’s the Difference: A Look at Consumer and Medical-Grade Wearables in Healthcare
Can both consumer and medical-grade wearables work together to fill healthcare’s care gaps? Dr. Sunil Kapur shares his insights.
What does Abbott’s Confirm Rx insertable cardiac monitor (ICM) and the latest Apple Watch have in common? The answer: more than you think, and perhaps not nearly enough. Today, consumer and medical-grade wearables may live in different markets, but they are aligning on a similar long-term objective—capturing critical data to improve outcomes.
“People want to
Read More
Digital Twin Technology: Should Healthcare Jump on the Bandwagon?
While healthcare is known for not jumping to conclusions very fast when investing in new technology, the industry starts to pick up the pace. Such “newcomers” as blockchain, IoT, VR, and AR get a well-deserved appraisal and become an integral part of next-gen custom healthcare software aimed at supporting personalized treatment and predictable patient outcomes across a wide range of conditions.
We anticipate that the digital twin technology has good chances to become the next addition to the
Read More
UM Spinout Fifth Eye Lands $11.5M for Early Warning Patient Deterioration System for Hospitals
Fifth Eye, a medical device software spinoff from the University of Michigan building clinical early warning patient deterioration system for hospitals has raised $11.5 million in Series A funding led by Arboretum Ventures and Cultivation Capital. Other participants in the round include were MINTS, the direct investing arm of the University of Michigan’s endowment, along with additional capital from previous investors Invest Michigan and 35 private angel investors.Why Clinicians Need A Better
Read More
Biotricity Awarded FDA 510(k) Clearance for its Biometric Remote Monitoring Device
Biotricity - a medical diagnostic and consumer healthcare technology company dedicated to delivering innovative, biometric remote monitoring solutions, that details how the Company has received its 510(k) clearance for its Bioflux device with the U.S. Food and Drug Administration (FDA). This latest 510(k) is the final FDA requirement needed for Biotricity to bring to market Bioflux in the U.S.Bioflux consists of a proprietary mobile ECG monitoring device and an ECG viewer software package, that
Read More