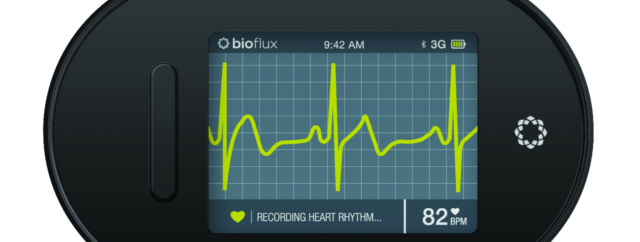
Biotricity – a medical diagnostic and consumer healthcare technology company dedicated to delivering innovative, biometric remote monitoring solutions, that details how the Company has received its 510(k) clearance for its Bioflux device with the U.S. Food and Drug Administration (FDA). This latest 510(k) is the final FDA requirement needed for Biotricity to bring to market Bioflux in the U.S.
Bioflux consists of a proprietary mobile ECG monitoring device and an ECG viewer software package, that enables physicians to remotely monitor and diagnose patients with cardiovascular coronary heart disease by detecting and transmitting probable arrhythmias, along with other diagnostic heart information. The remote monitoring solution can be utilized for early detection which the Company believes is an example of the growing shift in healthcare towards a preventative system.
Biotricity has started its first production run of the Bioflux solution. With large scale manufacturing already in place, the company is primed to begin mass production. “We are ready to hit the ground running,” said Waqaas Al-Siddiq, Founder and CEO of Biotricity. “With our manufacturing infrastructure fully developed, we expect to be able to bring the Bioflux solution to market imminently.”
Biotricity’s mission is to develop multiple medical-grade biometric remote monitoring solutions for a variety of chronic illnesses beginning with cardiac health. These medical grade devices are of particular importance to physicians, as they have become weary of the data provided by light-weight fitness trackers such as Fitbit. Biotricity’s approach is to combine a proprietary mobile ECG monitoring device and an ECG viewer software package that will enable physicians to remotely monitor and diagnose patients with cardiovascular disease and coronary heart disease. This is accomplished by detecting arrhythmias, using an accredited 24 hour, 7 day per week, ECG monitoring facility.
“We are incredibly excited about receiving our hardware 510(k) clearance as this is the final step needed before commercializing our first medical-grade solution,” said Al-Siddiq. “As we bring Bioflux to market, we intend to continue to develop other disease specific remote biometric technologies that can help diagnose, treat, and manage chronic diseases through clinically accurate and tailor-made solutions.”
