The American Cancer Society’s Cancer Statistics 2023 report highlighted a few major accomplishments within oncology, such as decreased cancer mortality rates and increased trial diversity. However, there is still much work to be done. Especially with the multiple types of new and ongoing challenges clinicians and staff members are facing - such as high turnover rates, decreased budgets and increased feelings of burnout - taking the necessary steps to enhance clinical trial procedures is crucial
Read More
Clinical Trial
ObvioHealth Launches Decentralized Clinical Trial for Migraine Treatment Device
What You Should Know:
- ObvioHealth and Mi-Helper, Inc. announce a partnership to conduct a decentralized clinical trial for a non-invasive neuromodulation device for the treatment of migraines. The randomized controlled trial will be fully remote, enabling data capture from home—where the device is intended to be used.
- Mi-Helper has been developed to meet a massive unmet need for effective, targeted, and drug-free pain management of migraines, a condition which impacts 1 billion
Read More
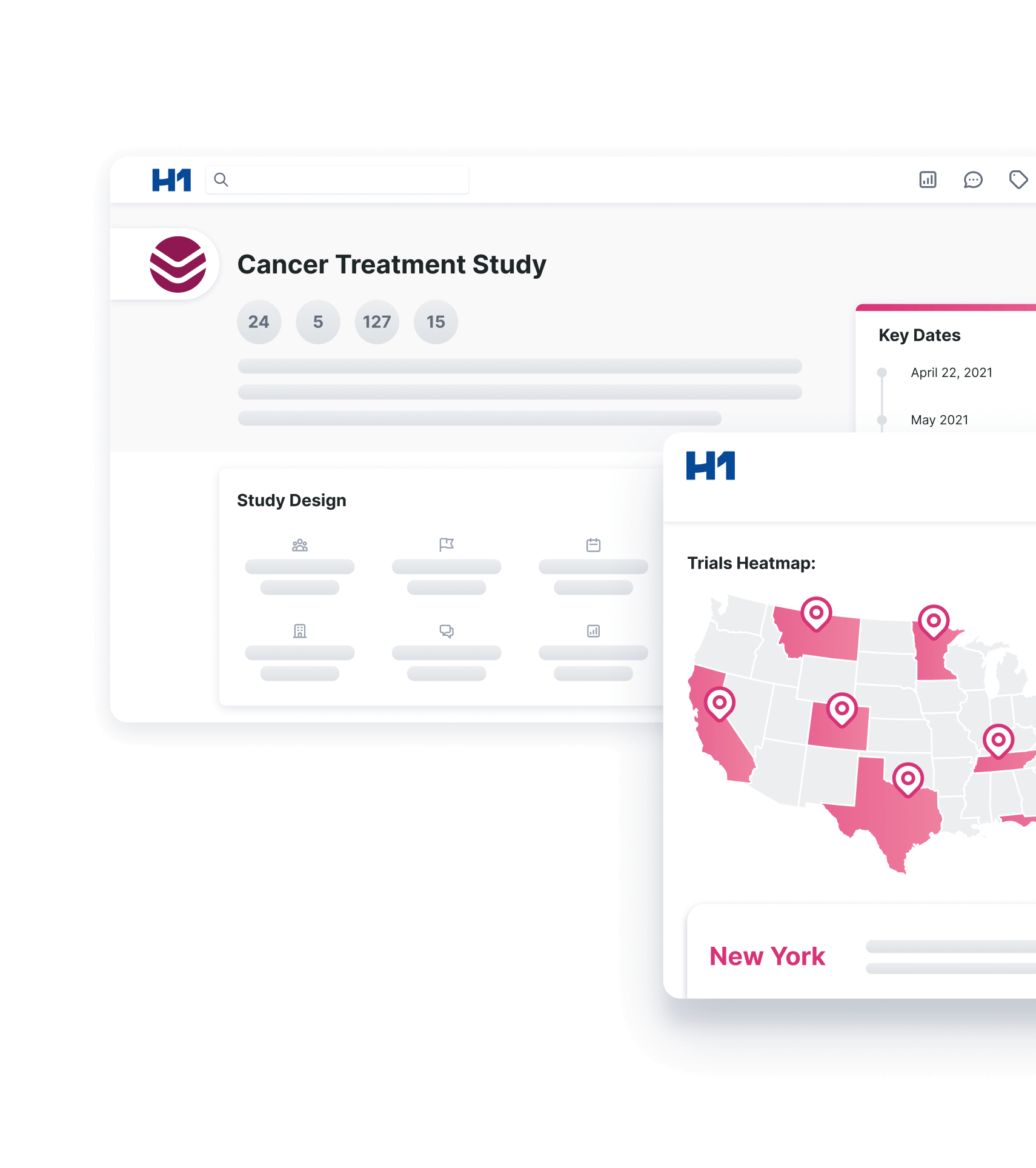
H1 Launches Advanced Diversity and Clinical Trial Performance Insights
What You Should Know:
- H1, the connecting force for global healthcare provider, clinical, science, and research information, today announced the availability of indication-level diversity insights in addition to institution, provider, and patient diversity data within its Trial Landscape solution.
- Additionally, H1 has successfully onboarded its first customer – a top 20 pharmaceutical company – onto the H1 Data Network, a new solution that aggregates sponsor-contributed site and
Read More
The Market Demand for Data-Driven Tech Solutions in Healthcare
The market demand for data-driven technology solutions in healthcare is on the rise. Today, one-third of the world's data is generated by the healthcare industry. This data is often complex and is coming from multiple different sources such as personal health information, claims data, clinical trial data, patient surveys, disease registries, population health statistics, wellness apps, and wearable devices. And so, the need for data management technologies continues to grow. In the Future Health
Read More
Why Clinical Data Research Needs a Makeover in 2023
Clinical research is only as good and thorough as the data that’s available — and we need to do a better job of making that data accessible for researchers.
Unfortunately, the majority of clinical data is based on the same clinical sites and makes conclusions off a limited range of data points. Clinical trials often exclude minorities, rare disease subtypes and other underrepresented populations.
Changing the way we gather and share clinical data isn’t easy. Hospitals, biobanks and
Read More
Tempus, Pfizer Partner to Advance Oncology Therapeutic Development
What You Should Know:
- Today, the AI-powered genetic testing and precision medicine company Tempus announced that it has signed a significant strategic agreement with Pfizer to more precisely gather insights that will inform novel drug discovery and development in oncology.
- Through this collaboration, Pfizer has access to Tempus’ AI-enabled platform and its library of de-identified, multimodal data to uncover insights that will power therapeutic development in oncology.
-
Read More
ObvioHealth Launches Digital Therapeutics API for Clinical Trials
What You Should Know:
- ObvioHealth launches proprietary application programming interface (API) tailored specifically for digital therapeutics (DTx) clinical trials.
- ObvioHealth’s API ensures tight integration between the digital therapeutic and the ObvioGo platform, providing sponsors with accurate data to correlate efficacy with adherence to the DTx. In addition, ObvioGo’s integration reduces participant task duplication because—in most cases—outcomes can be passively captured
Read More
Addressing the Diversity Challenge in Clinical Research Through Patient Advocacy
Diversity is paramount to the success of clinical research, both ethically and scientifically. Without adequate representation of racial and ethnic minorities, investigators lack an understanding of the safety and efficacy of novel treatments within those groups. From a scientific standpoint, an incomplete dataset hinders drug delivery and the development of breakthrough therapies – but from an ethical standpoint, underrepresentation creates a barrier to treatment options for populations that
Read More
Vysioneer & Pfizer Partner on AI-Powered Oncology Clinical Trials
What You Should Know:
- Vysioneer, a provider of applied AI for Oncology, today announced a data-sharing agreement with Pfizer, a global leader in pharmaceuticals. The agreement aims to lay the foundation for the application of AI in oncology clinical trials.
As part of the agreement, Vysioneer gains access to one of Pfizer's oncology clinical trial datasets to apply Vysioneer's advanced machine-learning techniques to facilitate the drug efficacy assessment process, thereby allowing Pfizer
Read More
5 Components of a Patient-Centric eCOA Strategy for Oncology Clinical Trials
In the US alone, an estimated 1.9 million new cases of cancer were diagnosed in 2022, positioning oncology as a key subject of clinical research. Throughout oncology trial development, it is important that stakeholders acknowledge that only patients can fully understand the impact of treatment on their lives. Regulators are now looking beyond clinical indications such as tumor size and delayed disease progression. When evaluating the risks and benefits of treatment, they want to know whether the
Read More