Consumables are often associated with low-cost, standardized products. This has often been the case with breathing system solutions used in ventilation. However, the COVID-19 pandemic has highlighted the importance of sufficient infection control in the hospital environment, subsequently uplifting demand for solutions that reduce infection risk. Vendors of all calibers grabbed this opportunity to expand market share, but questions remain on whether they will keep up in a crowded and
Read More
Life Sciences | News, Analysis, Insights - HIT Consultant
Benchling Acquires Overwatch Research to Accelerate Breakthroughs for Biopharma Companies
What You Should Know:
- Today, R&D Cloud pioneer Benchling announced it has acquired Overwatch Research to accelerate in vivo research.
- The acquisition will create the industry’s first comprehensive, cloud-native in vivo solution – a critical component of drug discovery and development – drastically accelerating the time it takes biopharma companies to bring products to market.
Digital Transforming R&D
In vivo studies are a critical component of drug discovery and
Read More
Sema4, BioSymetrics Partner to Advance Data-Driven Drug Discovery
What You Should Know:
- Today, Sema4, a leader in AI-driven clinical data intelligence, announced that they have partnered with BioSymetrics, to accelerate the advancement of drug discovery and new treatments for patients and pharmaceutical companies.
- Together the two will leverage Sema4’s Centrellis®, one of the largest and fastest-growing integrated health information platforms that includes approximately 12 million de-identified clinical records, and Elion, BioSymetrics’
Read More
Helping Clinical Trial Sites Improve Study Recruitment and Retention
The clinical trial industry is, once again, at an uncertain crossroads. And while it might be assumed that the outsized impact of the COVID-19 pandemic has resulted in the inability to recruit patients for trials, it is instead, a challenge generated, over time, by the industry itself. Thankfully, there is an opportunity to change course.
It’s no secret that research studies and clinical trial sites struggle with recruitment. In fact, the National Institutes of Health (NIH) states
Read More
Variantyx Secures $41.5M for Genetic Testing and Personalized Medicine
What You Should Know:
- Variantyx, a technology-driven precision medicine company providing advanced genomic testing for the rare genetic disorders, reproductive health, and precision oncology markets, today announced they have secured $41.5M in C-2 funding led by New Era Capital Partners and includes Peregrine Ventures, Robert Bosch Venture Capital, 20/20 HealthCare Partners, and Pitango HealthTech.
- Founded in 2014, the Variantyx proprietary whole genome sequencing and analysis
Read More
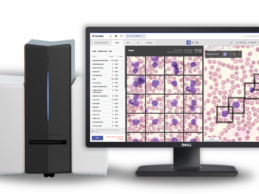
Scopio Secures $50M for AI-Powered Peripheral Blood Smear App
What You Should Know:
- Scopio Labs, provider of the world's first full-field digital cell morphology solution, today announced a $50M investment for its end-to-end hardware and software platform for digitizing, quantifying and analyzing hematology and other digital cytology samples.
- The Series C round included OurCrowd, Aurum Ventures, Mizrahi-Tefahot Bank Invest and Ilex Medical, with the participation of strategic investors operating in the field and existing
Read More
Medable Launches Decentralized Clinical Trials Partner Network
What You Should Know:
- Medable Inc., a Palo Alto, CA-based provider for patient-centered clinical trials, today launched the Medable Partner Network – uniting a diverse ecosystem of technology, service, data, site, and direct-to-patient partners that work together to accelerate deployment of decentralized clinical trials.
- The network includes new partnerships with Advanced Clinical, Cognizant, and CVS, and expanded relationships with Datavant, Oracle, Parexel, PPD, part of
Read More
Lucira Health Secures $80M to Expand COVID-19 Test Kit
What You Should Know:
- Lucira Health, Inc. (Nasdaq: LHDX), a medical technology company focused on the development and commercialization of transformative and innovative infectious disease test kits, today announced it has secured a debt facility of up to $80M with Hercules Capital, Inc. and Silicon Valley Bank.
- Lucira's COVID-19 testing platform produces lab-quality molecular testing in a single-use, consumer-friendly, palm-size test kit powered by two AA batteries. The FDA EUA
Read More
C2i Genomics, Twist Bioscience Partner to Develop Whole-Genome Cancer Reference Materials
What You Should Know:
- Today, Twist Bioscience and cancer genomics startup C2i Genomics announced a partnership to develop whole-genome cancer reference materials.
- This resource will provide diagnostic labs around the globe with the ability to better validate and monitor the quality of their whole-genome cancer screening and minimal residual disease (MRD) products for more accurate cancer detection.
C2i to integrate Twist’s library preparation kits into minimal residual
Read More
CVS Health Taps Medable to Bring Decentralized Clinical Trials to Pharmacies Nationwide
What You Should Know:
- Medable and CVS Health announced a strategic collaboration that will make clinical research more accessible to patients of all demographics. Using Medable’s decentralized trial platform available to CVS Health’s thousands of community MinuteClinics nationwide, most people will be within just five minutes of a decentralized clinical trial research site – finally busting the longstanding barrier to research for millions of Americans
- Where applicable, CVS Health will
Read More