- Healthy.io raises $60M led by Corner Ventures for its FDA 510(k) cleared smartphone-based ACR test to be used in the aid of diagnosing chronic kidney disease (CKD).
- The funding round will be used to accelerate Healthy.io’s global expansion and product development.
- Healthy.io’s solution allows immediate electronic medical record (EMR) connectivity through the automated smartphone scan.
Healthy.io, the fast-growing Israeli digital health company that has turned
the
Read More
Medical Devices | FDA | News, Analysis, Insights - HIT Consultant
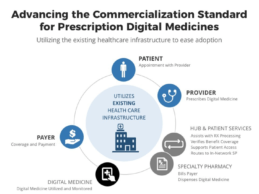
Cognoa, EVERSANA Partner to Advance the Commercialization Standard for Prescription Digital Medicines
- Cognoa and EVERSANA form a partnership that will enable prescription, dispensation, and reimbursement of Cognoa’s behavioral health products.
- Cognoa is developing digital medicine solutions to change the standard of care in pediatric behavioral health to improve the lifelong outcomes for children.
- Cognoa selected EVERSANA, a fully integrated and independent commercial services platform, to develop and manage a go-to-market strategy that ensures comprehensive market
Read More
ResApp Awarded CE Mark for Smartphone-Based Diagnostic Test for Respiratory Disease
ResApp Health, the developers of the world’s first smartphone-based diagnostic test for respiratory disease where one simply coughs into the phone and the machine learning-based algorithm diagnoses the underlying respiratory disease, has received CE Mark certification as a Class IIa medical device.Significance of CE Mark CertificationCE Mark certification indicates that ResAppDx-EU meets the essential requirements of all the applicable European regulations as a medical device and allows for the
Read More
OSF Ventures Joins $35M Funding Round for Handheld, Exo Ultrasound Device
OSF Ventures, the investment arm of OSF HealthCare has joined a $35M Series B funding round to support medical device startup Exo Imaging that’s developing a breakthrough handheld, AI ultrasound device that will allow healthcare providers to make critical, real-time diagnostic decisions.Performance Ultrasound PlatformFounded in 2015, Exo (pronounced “Echo”) is bringing diagnostic-grade medical imaging to the pocket of every caregiver and clinician worldwide. The company is developing an
Read More
Mercy Launches a Nationwide Real-World Evidence (RWE) Network to Pool De-Identified Patient Data
Mercy Technology Services (MTS), the information technology arm of St. Louis-based Mercy health system, has launched a nationwide real-world evidence network to pool clinical data for advanced analysis. The new initiative aims to create a new model of curated data sharing among providers, drug and device makers, regulators and more to power better patient care.MTS Real-World Evidence (RWE) Network BackgroundDeveloped in partnership with global tech giant SAP, MTS’ new network aims to tap into
Read More
What’s AI’s Holdup in Healthcare? The Answer Is In The Cloud
When you consider what technology has the most potential to transform healthcare over the next decade and beyond, no doubt your first thoughts turn to artificial intelligence (AI), machine learning (ML) and deep learning (DL). Already these technologies are showing promise in helping clinicians work faster and smarter, identifying trends, alerting us to signs that can predict and prevent diseases, and providing insights into potential treatments and cures.
But AI, ML, and DL alone cannot
Read More
What Is The Upside of Establishing Extended Reality As A Medical Device?
XR stands for extended reality. Basically, it's an umbrella term covering the VR (virtual reality), AR (augmented reality), and MR (mixed reality) technologies. All three are already involved or will be involved in healthcare. In the very near future, you will be able to “swipe” between all three within an application. Different combinations of the technologies will be used to provide specific health benefits, eventually all within a single platform.
It is sometimes hard to explain to people
Read More
UK Startup Flow Raises $1.5M for Brain Stimulation Headset, Therapy App
Flow, a London-based medical device startup using a brain stimulation headset and therapy app to treat depression has raised $1.5 million in seed funding led by Khosla Ventures. In Europe, Flow has been approved as a Class II medical device intended for use as a treatment for depression – and is the first approved treatment of its kind in Europe available to buy and use at home. The investment will be used to support Flow’s European rollout, introduce Flow to healthcare clinics, and fund
Read More
Procyrion Raises $30M to Support Clinical Trials of its Percutaneous Blood Pump Device
Procyrion, Inc., a Houston-based medical device firm developing the first catheter-deployed, intra-aortic pump initially for use in cardiorenal syndrome patients announced it has raised $30 million in Series D funding led by Bluebird Ventures. The round also includes participation from existing investors Fannin Partners, Scientific Health Development, the State of Texas, and an undisclosed strategic investor.The company is developing its AortixTM system, a percutaneous blood pump initially
Read More
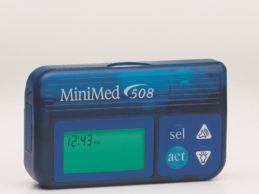
FDA Recalls Medtronic MiniMed Insulin Pumps for Potential Cybersecurity Risks
The U.S. Food and Drug Administration (FDA) is recalling certain Medtronic MiniMed insulin pumps due to potential cybersecurity risks and recommends that patients using these models switch their insulin pump to models that are better equipped to protect against these potential risks. To date, the FDA is not aware of any confirmed reports of patient harm related to these potential cybersecurity risks.Medtronic's MiniMed Insulin PumpsThe FDA is concerned that, due to cybersecurity vulnerabilities
Read More