The global home medical equipment market is experiencing explosive growth. Smart devices are being used to monitor and treat patients suffering from diabetes, cardiac disorders, and other medical conditions without exposing those patients to the risk of hospital-acquired infections. Numerous organizations, including the World Health Organization and the National Council of Aging, are promoting self-health management to give patients better outcomes.
This trend leverages digital technology to
Read More
Medical Devices | FDA | News, Analysis, Insights - HIT Consultant
Patching Problems? Securing Medical Device Software Postmarket
Medical device manufacturers face a conundrum. To meet recent FDA requirements, which were initially introduced a little over a year ago, they now need to quickly provide postmarket updates and patches for their devices to address cybersecurity vulnerabilities and ensure their ongoing safety and effectiveness.
However, medical device manufacturers know all too well that patching takes significant time and engineering resources, is difficult to accomplish for Class II and Class III medical
Read More
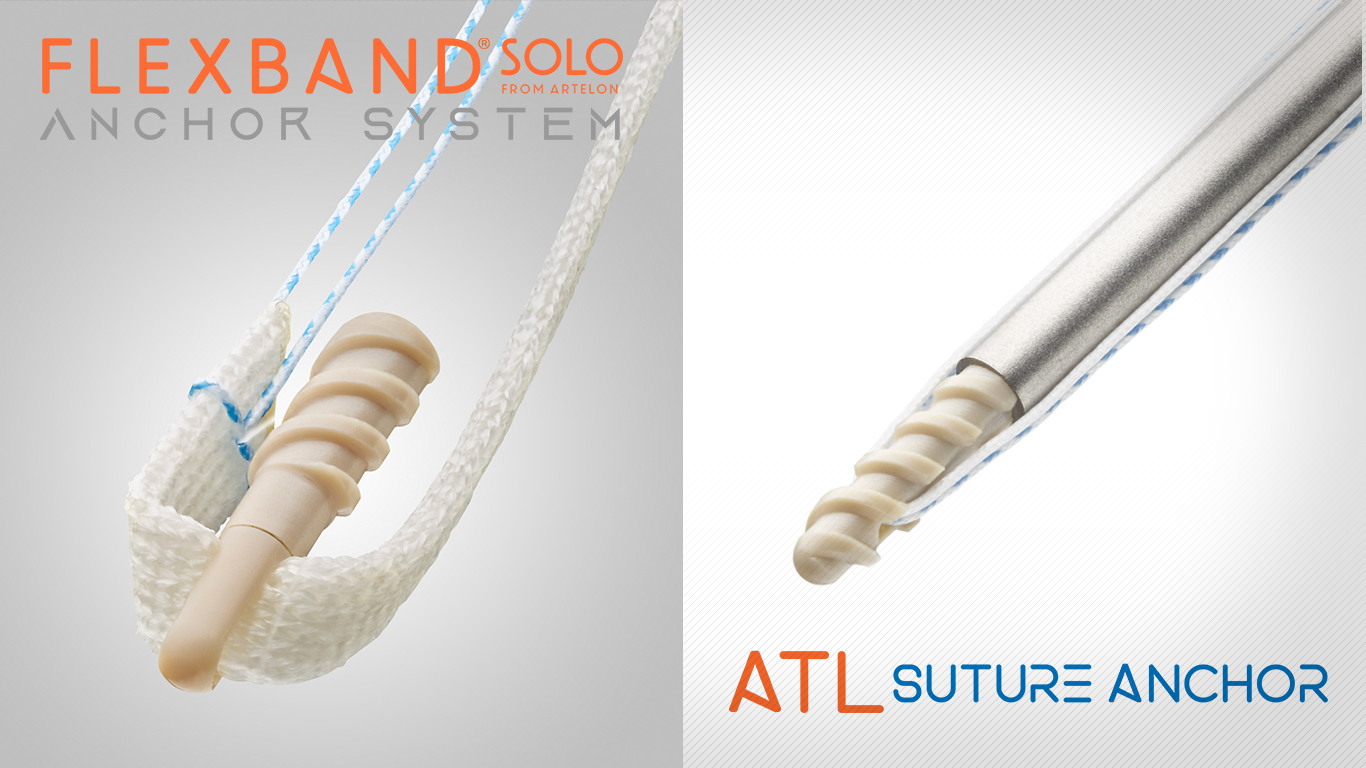
Artelon Secures $20M to Advance Surgical Treatment of Joint Instability
What You Should Know:
Artelon Inc., a privately held medical device company raises $20M in Series B funding, led by Vensana Capital with expected additional participation from existing investors. Artelon's Dynamic Matrix technology is a proprietary polymeric bio-textile for musculoskeletal soft tissue reconstruction designed to mimic the natural mechanical and biological properties of healing ligament tissue. It has been proven in clinical studies to protect the surgical repair
Read More
Patient Square Capital Forms Elevage Medical Technologies to Partner with Growth-Stage Medical Device Companies
What You Should Know:
Healthcare investment firm Patient Square Capital ("Patient Square") forms Elevage Medical Technologies ("Elevage"), a new portfolio company to support the growth of promising medical device companies by providing capital and deep strategic expertise.
Elevage will deliver financial and strategic growth support to a broad set of medical device technology companies that are at the forefront of transforming health care through cutting-edge devices that enhance health
Read More
Magenta Medical Raises $55M for World’s Smallest Heart Pump
What You Should Know:
Magenta Medical, the developer of the world’s smallest heart pump, announced today a $55M financing round led by global healthcare investment manager OrbiMed, with participation from existing investors New Enterprise Associates (NEA), Pitango VC, and ALIVE - Israel HealthTech Fund.The financing will be used, among other things, to advance the clinical programs of the company’s product in the United States toward its first FDA
Read More
Expectations For The Connected Care Business In The Years Ahead
Though we seldom see their use in our modern world and, even then, only in fiction, there was a time when it was common for people to actually use things like crystal balls and divining rods to try to uncover unknown yet valuable information. As unbelievable as it may seem, soothsayers peered into crystal balls aiming to help seekers look into the future for guidance, while prospectors would rely earnestly on divining rods as they attempted to locate underground riches of water or oil.
While
Read More
How AI Can Deliver Benefits in Healthcare Manufacturing and Patient Device Usage
According to Grand View Research, the global artificial intelligence (AI) in the healthcare market size was valued at USD 15.4 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 37.5% from 2023 to 2030, to reach an estimated USD 208.2 billion. That’s an impressive growth rate, indicating the expected value delivery to a mostly early-phase adoption of AI in the healthcare marketplace. With such aggressive predicted growth, many health industry professionals
Read More
MedCrypt Funds Medical Device Usable Security Research at Tufts University
What You Should Know:
- MedCrypt, Inc., a proactive cybersecurity solutions provider for medical device manufacturers, announced its financing of the School of Engineering for a Tufts University fellowship program that will support research focusing on the investigation of medical device security and threat modeling.
- MedCrypt acknowledges the vital role that evidence-based security practices play in the MedTech industry and recognizes the need to address the existing gaps. Additionally,
Read More
Measure What Matters: The 2023 Medical Device Benchmark Report
What You Should Know:
The COVID-19 pandemic put the medical device industry front and center, especially with the demand for essential products like diagnostic tests, ventilators, and respiratory assistant devices.A new report outlining the state of medical device service showcases problems the industry must overcome, purporting solutions powered by emerging technologies to overcome these obstacles and ensure service accuracy.
Understanding the Key Insights and Trends within the
Read More
Accenture Invests in Digital Twin Solution Virtonomy
What You Should Know:
- Today, Accenture Ventures announced it has made a strategic investment in Virtonomy, a provider of data-driven simulations that use existing patient data and digital twin technology to bring life-saving medical devices to market more quickly.
- Virtonomy’s digital twin simulation solution enables medical device manufacturers to build model patient virtual environments for device testing at a reduced cost and with less regulatory complexity.
Read More