- Sony announces FDA 510(k) clearance from the FDA for NUCLeUS™, the smart operating room platform, for sale in the U.S.
- NUCLeUS provides an enhanced, streamlined workflow for operating rooms and clinical spaces with direct access to imaging data from an easy-to-use central dashboard.
Sony Electronics has received FDA 510(k) clearance from the
Food and Drug Administration for the company’s NUCLeUS™
Operating Room, Imaging Management and Collaboration Control platform.
Proven in
Read More
FDA| Healthcare FDA Regulation | Policy, News, Analysis, Insights - HIT Consultant
FDA Clears Eko’s AFib, Heart Murmur Detection Algorithms for AI-Powered Stethoscope
- StartX company, Eko, announced today that the FDA has cleared Eko’s AFib and heart murmur detection algorithms, making it the first AI-powered stethoscope to screen for serious heart conditions. - Eko’s algorithms alert clinicians to the presence of heart murmurs and atrial fibrillation (AFib) during the physical exam, converting the classic stethoscope into a powerful early detection tool. Eko, a digital health company applying artificial intelligence (AI) in the fight against heart disease,
Read More
BioIntelliSense Awarded FDA 510(k) Clearance for On-Body Sensor for Scalable Remote Care
- BioIntelliSense announces FDA clearance of the BioSticker, the first single-use medical device enabling 30 days of continuous vital signs monitoring. - The FDA-cleared BioSticker medical device is designed to be discreetly worn on the upper left chest for effortless remote data capture and a simplistic “stick it on and forget it” patient experience. BioIntelliSense, Inc., a continuous health monitoring and clinical intelligence company, today announces the U.S. commercial launch of its
Read More
Do We Know When Medical Devices Fail?
Since 2015, the FDA and the US Department of Homeland Security have been releasing warnings about products that due to their vulnerabilities threaten patient safety. This includes MRI machines and drug infusion pumps that supply patients with a wide diversity of drugs, including insulin, antibiotics, chemotherapy drugs, and pain relievers. The interconnectivity of smart devices with medical clinical systems leaves them vulnerable to security breaches just like any other networked computing
Read More
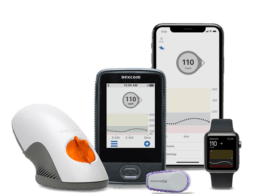
Livongo Integrates With Dexcom’s G6 Continuous Glucose Monitoring System
- Livongo announced a data integration partnership with Dexcom to offer Livongo Members the ability to sync data from their Dexcom G6 Continuous Glucose Monitoring System with the Livongo applied health signals platform. - As part of the integration, Livongo can now combine Dexcom CGM data with rich Member data from Livongo’s connected devices to deliver health insights that address the whole person.Livongo, an Applied Health Signals company has partnered with Dexcom, Inc. a provider of
Read More
3 AI Trends for 2020: Moving Beyond Dazzle to High-Impact Collaboration
From the first artificial skin to driverless cars that navigate busy streets with ease, 2019 brought a dazzling array of artificial intelligence (AI) discovery. In 2020, the progress made toward AI-fueled collaboration will be as critical as AI innovation—especially in healthcare.
We’ve reached a point where AI has proven its utility. In radiology, for example, AI is well-oriented to interpret diagnostic imaging studies, with FDA approval given to AI algorithms that diagnose a collapsed lung or
Read More
3 Healthcare Cybersecurity Trends to Watch in 2020
The healthcare industry frequently struggles with data breaches and other cybersecurity incidents. That's likely because cybercriminals know the value of medical data. It's also problematic that healthcare information often gets passed between multiple parties and organizations, some of which may have insufficient security practices.
Attacks from malicious actors aren't ceasing, which means healthcare cybersecurity must remain a priority in 2020. But, what, specifically, should parties
Read More
4 Digital Health Tools for Combating Opioid Addiction in 2020 & Beyond
The human and economic statistics surrounding the opioid epidemic are staggering. According to the U.S. Department of Health and Human Services, 11.4 million people have misused prescription opioids, and more than 130 people die daily from opioid-related drug overdoses.Moreover, the Society of Actuaries found that the total economic burden of the opioid crisis in the United States from 2015 to 2018 was at least $631 billion. The organization attributed nearly one-third of that cost to extra
Read More
Top 60 Health IT/Digital Health Mergers & Acquisitions in 2019
- From Google's acquisition of Fitbit to Philips acquisition of Carestream Health's health IT business, here is a look at some of the biggest health IT/digital health mergers & acquisitions in 2019 by each quarter.
Q1 M&A Activity
Johnson & Johnson Acquires Auris Health for $3.4B to Expand Digital Surgery Portfolio
Johnson & Johnson acquiresAuris Health, a developer of robotic technologies for $3.4 billion in cash. Auris Health is a privately-held developer of
Read More
Augmedics Secures FDA 510(k) Clearance for Augmented Reality Guidance System for Surgery
- Augmedics announced U.S. Food and Drug Administration (FDA) 510(k) clearance and the U.S. launch of its groundbreaking xvision Spine system (XVS), the first AR guidance system to be used in surgery.
- xvision Spine allows surgeons to visualize the 3D spinal anatomy of a patient during surgery as if they had “x-ray vision,” and to accurately navigate instruments and implants while looking directly at the patient, rather than a remote screen.
Today, Augmedics announced U.S. Food and Drug
Read More