What You Should Know: Quest Diagnostics has reached an agreement to acquire Haystack Oncology an early-stage oncology company focused on minimal residual disease (MRD) testing to aid in the early, accurate detection of residual or recurring cancer and better inform therapy decisionsUnder the terms of the agreement, Quest will pay $300 million in cash at closing, net of cash acquired, and up to an additional $150 million on achieving future performance
Read More
lung
Report: Prior Authorization for Biomarker Testing Leads to Treatment Delays For Cancer Patients
What You Should Know: Biomarker testing is a necessary tool in the advancing world of precision cancer treatment. According to a new survey released by CancerCare, a leading national cancer support organization, biomarker testing helped doctors tailor therapy for nearly all the respondent patients (93%) whose cancers were tested over the past three years. Two in 10 cancer patients (20%) surveyed were able to avoid unnecessary chemotherapy and/or radiation and 10% became eligible for
Read More
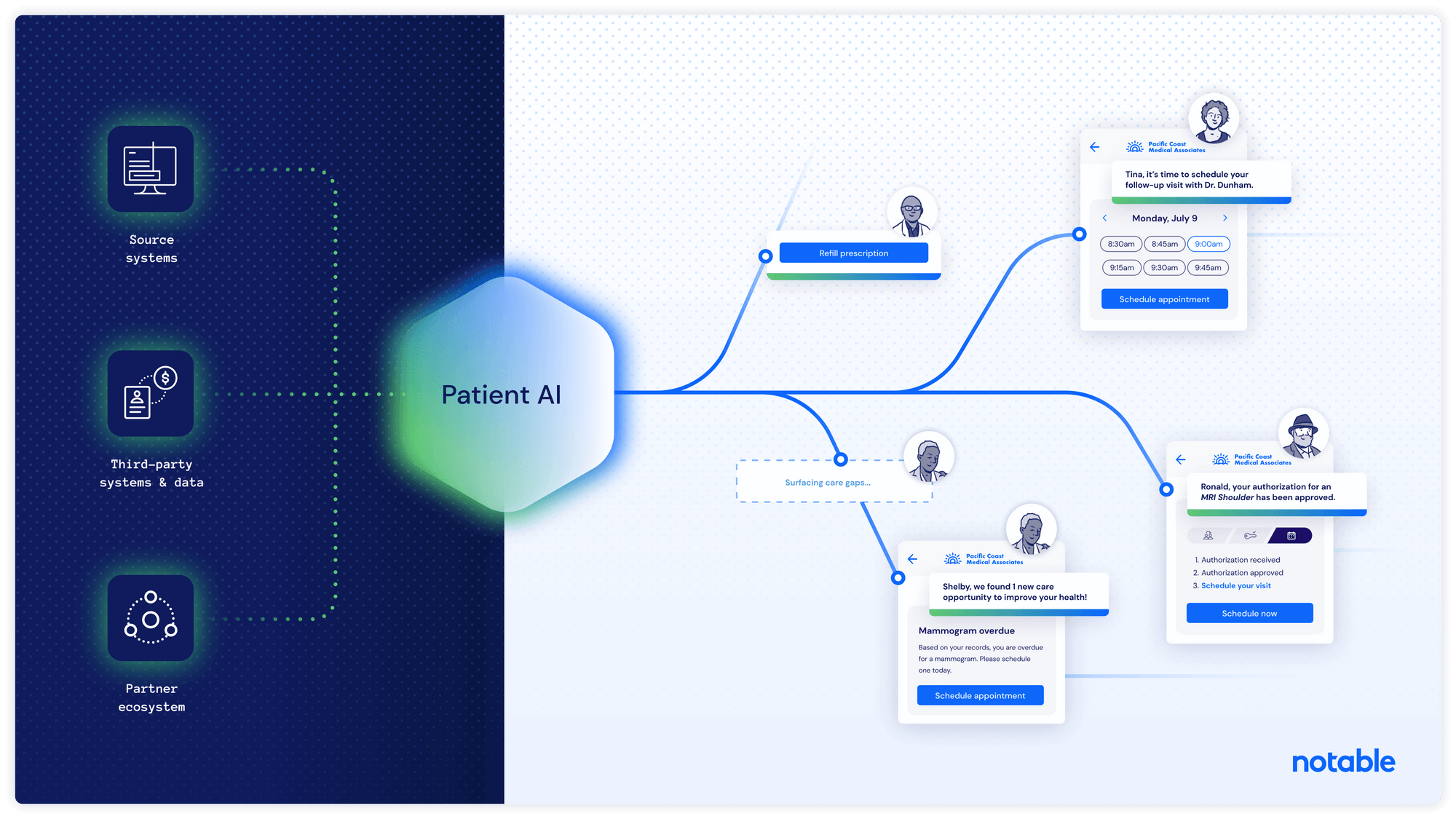
Notable Leverages GPT to Automate Patient Front Office Experience
What You Should Know: Notable, an intelligent automation company for healthcare, launches Patient AI, a solution leveraging large language models (LLMs) and GPT (the technology powering ChatGPT) to bring personalization at scale to healthcare.Using GPT to scan existing clinical documentation, this tech can detect missed diagnoses, identify lapsed insurance cards, incorrect addresses, eligible clinical trials, or costly care gaps in real-time across an entire patient population. How
Read More
Biostage Raises $6M to Advance Clinical Trials
What You Should Know: Biostage, Inc., a cell-therapy biotechnology company with successful first-in-human experience in treating esophageal cancer and FDA approval to commence a clinical trial of the Biostage Esophageal Implant raises $6M from new and existing investors in a private placement of its shares of common stock.The funds will be used to accelerate the clinical development of Biostage’s lead product candidate, the Biostage Esophageal Implant, or BEI. The FDA has approved a
Read More
AI-Enabled Butterfly Network Lung Tool Receives FDA Clearance
What You Should Know: - Today, Butterfly Network announced it received 510(k) clearance from the FDA for a groundbreaking AI-enabled tool named AI-enabled Auto B-line Counter that will help physicians assess adults’ lungs and accelerate providers’ abilities to make informed treatment decisions. Butterfly used data inputs from hundreds of sites across the country to train and develop its AI algorithms, offering potential for a broad and diverse range of age, gender, body mass index, ethnicity,
Read More
Protai Raises $20M to Build an Oncology Drug Discovery Pipeline
What You Should Know: - Protai, a proteomics and AI-powered drug discovery startup revolutionizing the way new drugs are discovered, today announced a $12 million extension of its seed round, bringing the total amount to $20M. - The round includes existing investors Grove Ventures and Pitango Healthcare and was joined by Copenhagen-based Maj Invest Equity Fund. The additional funding will be used to build Protai’s oncology drug discovery pipeline, expand data
Read More
TytoCare Receives FDA Clearance for AI-Powered Tyto Insights for Wheeze Detection
- TytoCare, a virtual care company enabling accessible, high-quality primary care from home, today announced that it received FDA clearance for its Tyto Insights for Wheeze Detection, paving the way for its rollout in the US. - The wheeze detection algorithm expands the company’s existing AI-powered Tyto Insights™ smart diagnostic capabilities, filling the quality gaps currently experienced with traditional telehealth and alleviating challenges imposed by the ongoing shortage of healthcare
Read More
Eko Launches Cardiac Disease Detection Platform, SENSORA
What You Should Know: - Eko, a digital health company applying artificial intelligence (AI) in the fight against heart and lung disease launches the SENSORA™ Cardiac Disease Detection Platform. - SENSORA™ currently features AI that objectively identifies structural murmurs, a sign of valvular heart disease, and Care Pathway Analytics software that provides downstream visibility and metrics of the patient journey through the healthcare system. Eko SENSORA Cardiac Disease Detection
Read More
Q/A: Oatmeal Health Co-Founder Talks AI-Enabled Cancer Screening for the Underserved
Today, cancer is the second leading cause of death in the United States. Sadly, cancer disparities exist, with racial/ethnic minority, low-income, and uninsured populations suffering the greatest burden. That’s why routine cancer screening is critical to addressing cancer disparities as they have the potential to greatly reduce both incidence and mortality rates. To address this, Federally qualified health centers (FQHCs) are funded by the Health Resources and Services Administration to provide
Read More
Aluna Raises $15.3M for Lung Health Management Platform
What You Should Know: - Aluna, the award-winning lung health management platform, recently announced it has completed a $15.3 million Series B round of financing to continue growing its solution among doctors and patients managing asthma, cystic fibrosis and COPD. - Aluna’s AI-enabled respiratory management platform allows patients to transmit data to their doctor by blowing into their innovative spirometer daily. The patient-facing app gives patients an easy way to collect symptoms,
Read More