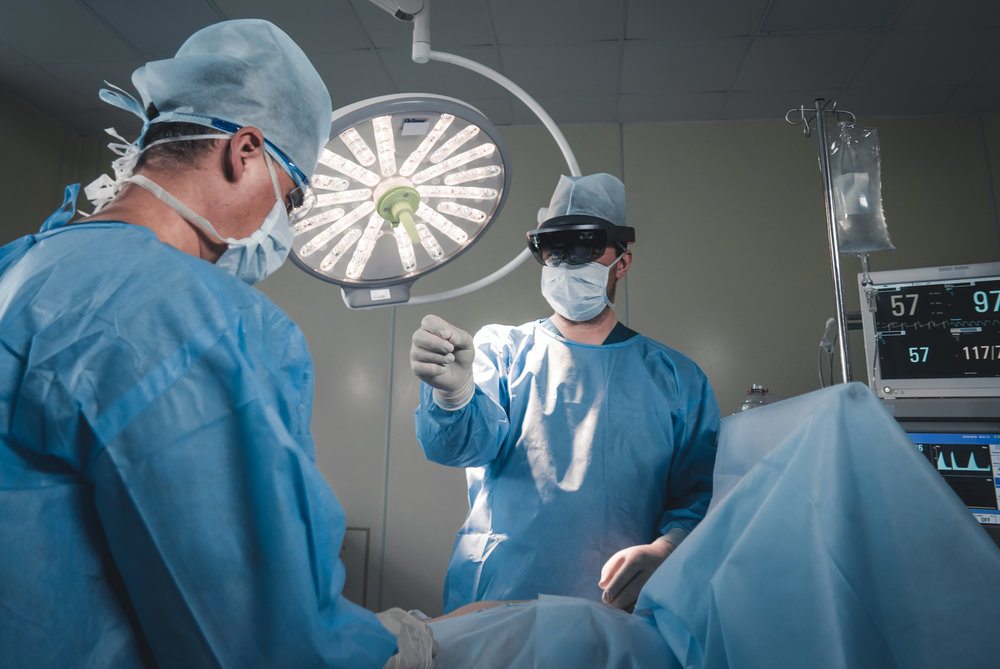
Medivis, a New York City-based medical technology company harnessing augmented reality and artificial intelligence to advance surgical visualization has received 510(k) clearance for clinical use in the operating room by the U.S. Food and Drug Administration (FDA).
Most medical procedures are performed relatively blindly, with surgeons having to reconstruct slices of 2D imaging data in the “mind’s eye” to make it actionable. Founded by neurosurgeon Osamah Choudhry, MD and radiologist Christopher Morley, MD, the enterprise SurgicalAR platform integrates the latest advancements in augmented reality, artificial intelligence, and computer vision to advance surgical visualization. The platform allows operators to have superior understanding, confidence, efficiency, and precision for every patient.
“Holographic visualization is the final frontier of surgical imaging and navigation,” said Dr. Osamah Choudhry, neurosurgeon & CEO of Medivis. “The surgical world continues to primarily rely on two-dimensional imaging technology to understand and operate on incredibly complex patient pathology. “
This announcement comes on the heels of significant company momentum, including strategic partnerships with Verizon and Microsoft. Earlier this year, the company came out of stealth with $2.3M in funding, led by Initialized Capital. The company also recently released their AnatomyX platform for AR medical training.
