What You Should Know:
- PathAI, a global leader in artificial intelligence (AI) and digital pathology solutions, today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its digital pathology image management system, AISight® Dx, for use in primary diagnosis in clinical settings.
- The FDA clearance builds upon the platform's initial 510(k) clearance for AISight Dx(Novo) in 2022, demonstrating PathAI’s ongoing commitment to innovation and
Read More
FDA clearance 510k
Withings Sleep Rx Receives FDA Clearance for Home Sleep Apnea Testing
What You Should Know:
- Withings, a global leader in connected health, has announced that its Sleep Rx sleep apnea device has received clearance from the U.S. Food and Drug Administration (FDA).
- Sleep Rx represents a significant advancement in the diagnosis of sleep apnea, offering a more convenient and accurate alternative to traditional in-lab testing.
Addressing the Underdiagnosis of Sleep Apnea
Sleep apnea is a serious condition that affects millions of people
Read More
Philips IntelliSite Pathology Solution Receives 510(k) Clearance
What You Should Know:
- Philips IntelliSite Pathology 5.1 has received 510(k) clearance from the FDA, solidifying its position as the most widely deployed digital pathology solution for primary diagnosis globally.
- With over 300 pathology labs already implementing Philips' solution, and over 20 labs achieving complete digital workflows, the success of this technology is evident.
Investing in the Future of Cancer Care
By empowering collaboration, boosting efficiency, and
Read More
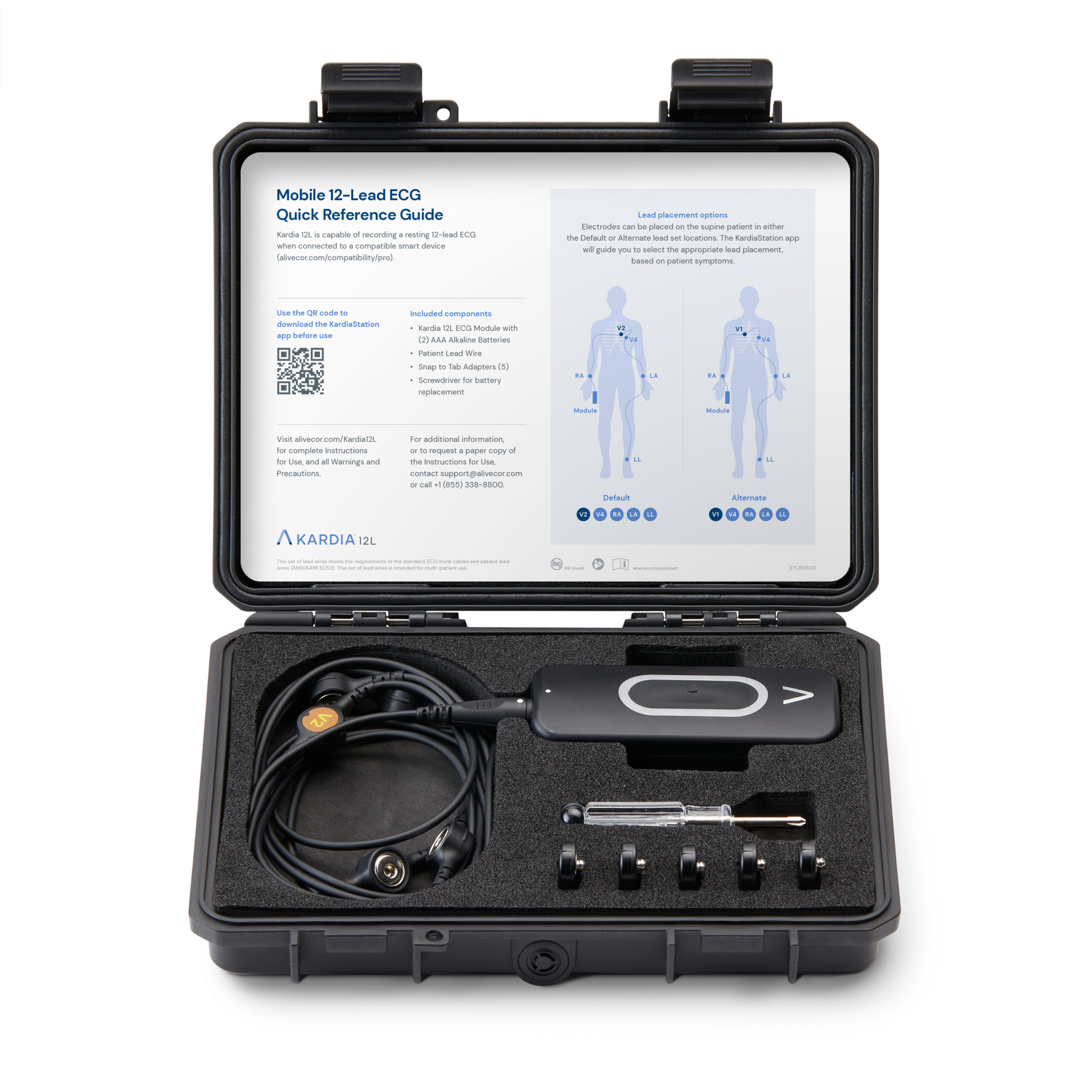
FDA Clears AliveCor’s Heart Attack-Detecting AI Kardia 12L ECG System
What You Should Know:
- AliveCor, a leader in AI-powered cardiology, has received FDA clearance and launched a groundbreaking innovation – the Kardia™ 12L ECG System with KAI™ 12L AI technology.
- AliveCor's Kardia 12L ECG System signifies a major advancement in cardiac diagnostics. By combining cutting-edge AI technology with a portable and user-friendly design, the system empowers healthcare professionals to deliver faster, more accurate, and more accessible cardiac
Read More
WearLinq Acquires AMI Cardiac Monitoring, Raises $6.7M for FDA-Cleared 6-Lead ECG Device
What You Should Know:
- WearLinq, a leader in wearable health monitoring, announced a strategic acquisition of AMI Cardiac Monitoring, LLC, a seasoned player in ambulatory cardiac monitoring.
- The acquisition, coupled with a recent $6.7M funding round, positions WearLinq to significantly expand access to their innovative eWave 6-lead ECG monitor and revolutionize the fight against atrial fibrillation (AFib), which recently received FDA 510(k) clearance.
Tackling the
Read More
Implicity Launches FDA-approved Heart Failure Prediction Algorithm, SignalHF
What You Should Know:
- Implicity, a leader in remote patient monitoring and cardiac data management, has received FDA 510(k) clearance for SignalHF1, a groundbreaking new algorithm within their remote monitoring solution.
- SignalHF1 algorithm empowers early detection and intervention, paving the way for improved patient outcomes and a brighter future for cardiac care.
Leveraging Big Data for Better Heart Care
Implicity holds a unique distinction as the first private
Read More
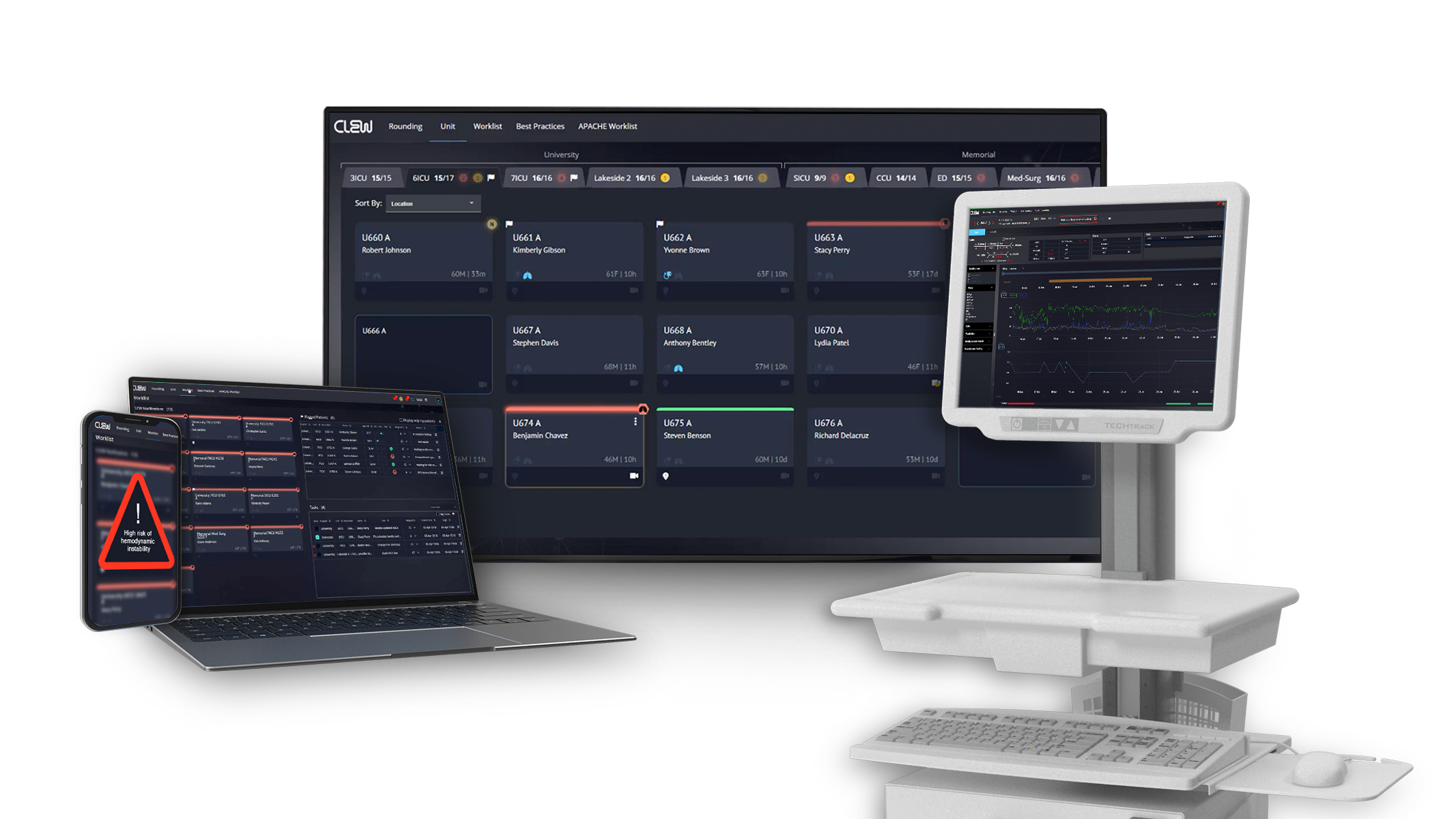
CLEW Medical Scores FDA Clearance for Next-Gen AI-Powered Patient Deterioration Prediction
What You Should Know:
- CLEW Medical, a provider of AI-driven clinical surveillance and predictive analytics, has achieved a breakthrough with FDA 510(k) clearance for its second-generation AI models for predicting patient deterioration.
- The FDA clearance builds upon their pioneering achievement of being the first FDA-cleared Class II medical device for this purpose in 2021.
Rigorous Testing for FDA Approval
Obtaining FDA clearance signifies that CLEW's AI models have
Read More
Philips Receives FDA 510(k) Clearance on Image-Guided Surgical Therapy
What You Should Know:
- Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced its Philips Zenition 30 mobile C-arm has received FDA 510(k) clearance, making image-guided surgical procedures available to more US patients at lower cost.
- By giving surgeons greater flexibility, control, and
Read More
AI Breakthrough for Lung Disease: Thirona’s LungQ Receives FDA Clearance
What You Should Know:
- Thirona, a pioneer in AI-powered lung analysis, received a major boost for its cutting-edge technology LungQ™ with the U.S. Food and Drug Administration (FDA) 510(k) clearance for its latest update, v3.0.0.
- This FDA clearance paves the way for American hospitals to now harness the software's advanced capabilities, marking a significant step forward in lung disease diagnosis and treatment.
LungQ 3.0.0: Precision Navigation for Lung Interventions
This latest
Read More
FemTech: Mosie Baby Awarded FDA Clearance for At-Home Intravaginal Insemination
What You Should Know:
Mosie Baby, a pioneering at-home fertility care company, has secured FDA Class II clearance for its Mosie Baby Kit making it the first and only FDA-cleared over-the-counter kit for use in intravaginal insemination (IVI).The kit was created to support those who are unable to conceive with intercourse or for whom intercourse is not an option. Following its FDA 510k Class II clearance, Mosie Baby looks forward to expanding access to its Mosie Baby Kit which was
Read More