Flow, a London-based medical device startup using a brain stimulation headset and therapy app to treat depression has raised $1.5 million in seed funding led by Khosla Ventures. In Europe, Flow has been approved as a Class II medical device intended for use as a treatment for depression – and is the first approved treatment of its kind in Europe available to buy and use at home. The investment will be used to support Flow’s European rollout, introduce Flow to healthcare clinics, and fund
Read More
Medical Grade Wearables
What’s the Difference: A Look at Consumer and Medical-Grade Wearables in Healthcare
Can both consumer and medical-grade wearables work together to fill healthcare’s care gaps? Dr. Sunil Kapur shares his insights.
What does Abbott’s Confirm Rx insertable cardiac monitor (ICM) and the latest Apple Watch have in common? The answer: more than you think, and perhaps not nearly enough. Today, consumer and medical-grade wearables may live in different markets, but they are aligning on a similar long-term objective—capturing critical data to improve outcomes.
“People want to
Read More
Flow Launches Medical-Grade Headset, Therapy App for Depression
Flow, a medical device company launches a medication-free treatment for depression comprising a brain stimulation headset and therapy app. In Europe, Flow has been approved as a Class II medical device intended for use as a treatment for depression - and is the first approved treatment of its kind in Europe available to buy and use at home.Flow BackgroundBased in Sweden, Flow was developed by clinical psychologist Daniel Mansson and neuroscientist Erik Rehn, together with a team of prominent
Read More
Biolinq Lands $4.75M to Expand Biosensor Patch for Continuous Glucose Monitoring
Biolinq, a San Diego, CA-based digital health company developing a minimally-invasive electrochemical biosensor patch for continuous glucose monitoring (CGM) has raised $4.75 million in Series A funding led by the JDRF T1D Fund, Aphelion Capital and LifeSci Venture Partners. Existing investors in the round include M Ventures and Hikma Ventures, the corporate venture capital arm of Hikma Pharmaceuticals. The latest round brings Biolinq’s total Series A funding to $15 million.Inception of
Read More
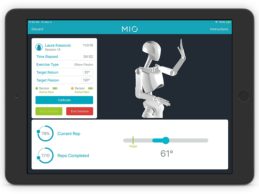
MbientLab Launches Wearable Sensor Solution for Physical & Occupational Therapists
MbientLab, a company building the next generation of sensors and tools for the healthcare industry, announced the availability of its MIOTherapy (MIO) – the first wearable technology platform that integrates the effectiveness of traditional physical therapy with smart sensors, therapeutic exercises, games, and 3D visualization technology to personalize and improve outpatient rehabilitation and accelerate recovery.Wearable Sensor Technology to Improve Outcomes of TherapyUsing a unique combination
Read More
Qardio Integrates Remote Monitoring Solution with Greenway Health EHR Solution
Qardio, today announced it has remote monitoring platform, QardioMD is integrated with Greenway Health’s electronic health record (EHR) and practice management solutions. QardioMD platform offers Qardio’s medical grade products and remote monitoring solutions to doctors, to help manage their patients’ health more efficiently while making billing for such care easier. The QardioMD remote monitoring solution is available to Greenway Health doctors via Greenway Marketplace at
Read More
Medical Wearables: How Next Generation Devices Will Change Healthcare
Medical patient monitoring devices are poised to disrupt the healthcare industry. According to Markets and Markets, the global market for medical wearable devices is projected to reach $12.1 billion by 2021, with the United States representing the largest market worldwide. With the ability to enhance the healthcare system by aiding in the remote monitoring of patients, wearables provide real-time access to health records and provide quicker diagnosis and treatment of conditions.They are
Read More
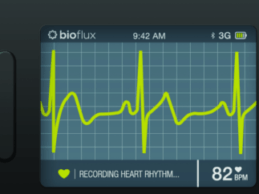
Biotricity Awarded FDA 510(k) Clearance for its Biometric Remote Monitoring Device
Biotricity - a medical diagnostic and consumer healthcare technology company dedicated to delivering innovative, biometric remote monitoring solutions, that details how the Company has received its 510(k) clearance for its Bioflux device with the U.S. Food and Drug Administration (FDA). This latest 510(k) is the final FDA requirement needed for Biotricity to bring to market Bioflux in the U.S.Bioflux consists of a proprietary mobile ECG monitoring device and an ECG viewer software package, that
Read More
VitalConnect Raises $38M to Launch Real-Time Wearable Biosensor In Hospitals Nationwide
VitalConnect, Inc., a provider of wearable biosensor technology for wireless monitoring in hospital and remote patient populations has raised $38 million in Series C funding led by MVM and Baxter Ventures. This funding will help VitalConnect in launching the VitalConnect Platform and VitalPatch wearable biosensor in hospitals and outpatient settings across the nation.Founded in 2011, VitalConnect combines the most sophisticated biosensor - the VitalPatch - with its comprehensive software
Read More
VitalConnect Lands $33M to Commercialize Medical-Grade Wearable Biosensor Platform
VitalConnect, a San Jose, CA-based provider of medical-grade wearable biosensor systems has raised $33 million in Series C funding led by new investors MVM Life Science Partners and Baxter Ventures, the venture arm of Baxter International. The company plans to utilize the funding to commercialize the VitalConnect Platform and make significant strides in changing the way patient monitoring is utilized in hospitals and at home. As part of the latest funding round, Dr. Stephen Reeders, MVM founder,
Read More