Podimetrics, a care management company with the leading solution to help prevent costly and deadly diabetic foot ulcer (DFUs) has raised $13.4M in Series B funding round from investors Rock Health, Norwich Ventures, and Scientific Health Development.Impact of Diabetic Foot Ulcers for PatientsDiabetic foot ulcers are a major complication of diabetes and are one of the most devastating and costliest diabetes complications -they can lead to debilitating amputations that cost on average $100k. In
Read More
FDA| Healthcare FDA Regulation | Policy, News, Analysis, Insights - HIT Consultant
Velano Vascular Raises $10M for FDA-Cleared Needle-Free Blood Draw Device
Velano Vascular, a San Francisco, CA-based vascular access technology innovator today announced that it has raised $10 million from strategic investor Intermountain Healthcare following the system’s rollout of the PIVO™ needle-free blood collection technology. The funding also included participation from Becton Dickinson’s former Chairman and CEO Edward Ludwig; notable entrepreneur, investor, and health sector philanthropist Marc Benioff; Baxter International’s former Chairman and CEO Robert
Read More
FDA Grants Breakthrough Designation to RenalytixAI for AI-Enabled Diagnostic for Kidney Disease
RenalytixAI, a developer of artificial intelligence-enabled clinical diagnostics for kidney disease, today announced that it has been granted Breakthrough Device designation by the U.S. Food and Drug Administration (FDA), for its lead diagnostic, KidneyIntelX™. This is the first such designation for an AI-enabled diagnostic for kidney disease publicly announced by any company.FDA Breakthrough Devices Program OverviewThe FDA’s Breakthrough Devices Program is a voluntary program for certain
Read More
RefleXion Medical Receives $60M Loan for Biology Guiding Radiotherapy Machine
RefleXion Medical, Inc., a biotargeting oncology company developing the first and only biology-guided radiotherapy (BgRT) machine* for targeted cancer treatment has received a $60M senior secured term loan from Oxford Finance LLC. The company plans to use the loan to support the completion of the FDA clearance process for its biology-guided BgRT system and its subsequent commercial launch.
Building from Positron
Emission Tomography
Positron emission tomography (PET) is an established
Read More
FDA Approves Blockchain/IoT Pilot to Track Specialty Prescription Drugs Across 3 States
The U.S. Food and Drug Administration (FDA) has approved a multi-regional pilot studying the use of blockchain-enabled data technology to track and verify specialty prescription drugs, to ensure safety, enhance value and improve health outcomes.Blockchain Pilot Consortium & Implementation TimelineThe pilot consortium is comprised of Rymedi,Temptime/Zebra, Indiana University Health, WakeMed Health & Hospitals, Good Shepherd Pharmacy and its RemediChain project, as well as the Center for
Read More
Med Student Develops Mobile Device Cover to Bring Health Apps into Sterile Settings
A medical student in the Michigan State University College of Osteopathic Medicine has announced the public launch and availability of the CleanCase sterile mobile devices cover, the first device-specific, fully FDA-complaint product on the market that allows surgeons to safely bring mobile devices into the sterile surgical field. The CleanCase cover, which was developed with investment from Red Cedar Ventures and Quantum Medical Concepts, uses patented technology to allow surgeons full mobile
Read More
Smartphone Detection of Chronic Kidney Disease Could Save NHS $877M Over 5 Years
Healthy.io, an Israeli-based provider of FDA-cleared smartphone-based urinalysis has announced the results from its early detection of chronic kidney disease (CKD) pilot study with 2,000 NHS patients with diabetes. The pilot found improved test compliance (raising responses to 72% among patients who had previously not responded to testing reminders) and discovered previously undiagnosed cases of CKD (10 percent of those who took the test).Financial Impact of CKD for the NHSWith a staggering
Read More
GoCheck Kids Nabs $6M for iPhone App That Detects Amblyopia in Pre-Verbal Children
GoCheck Kids, the creators of an FDA-registered and CE certified iPhone app that screens for amblyopia in pre-verbal children used by over 4,500 pediatricians announced it has raised $6M in Series B funding led by FCA Venture Partners. The investment will be used to add artificial intelligence capabilities with Apple’s CoreML and ARKit and accelerate further electronic health record (EHR) integrations.
Amblyopia: The Leading Cause of Vision Loss in U.S. Children
According to the CDC,
Read More
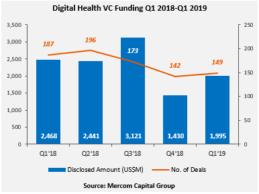
Mercom: Global Digital Health/Health IT VC Funding Tops $2B in Q1 2019
Global venture capital (VC) funding in digital health/health IT, including private equity and corporate venture capital, reach $2 billion in funding in Q1 2019, according to market research firm Mercom Capital. The Q1 2019 Digital Health (Healthcare IT) Funding and M&A Report reveals $2B in funding across 149 deals compared to $1.4B across 142 deals in Q4 2018. VC funding in Q1 2019 was down 19% compared to the same quarter of last year (Q1 2018) when nearly $2.5 billion was raised in 187
Read More
Medidata Launches New Life Sciences Company, Acorn AI
Life sciences digital transformation company Medidata, today announced the launch of Acorn AI, a new company designed to provide actionable insights across the entire drug continuum – from R&D to commercialization. In addition, the company has appointed Rama Kondru, Ph.D., as the chief information officer for Medidata and Acorn AI’s first CIO to lead the company’s enterprise data strategy, architecture and future platform development – all helping the company to accelerate the creation of
Read More