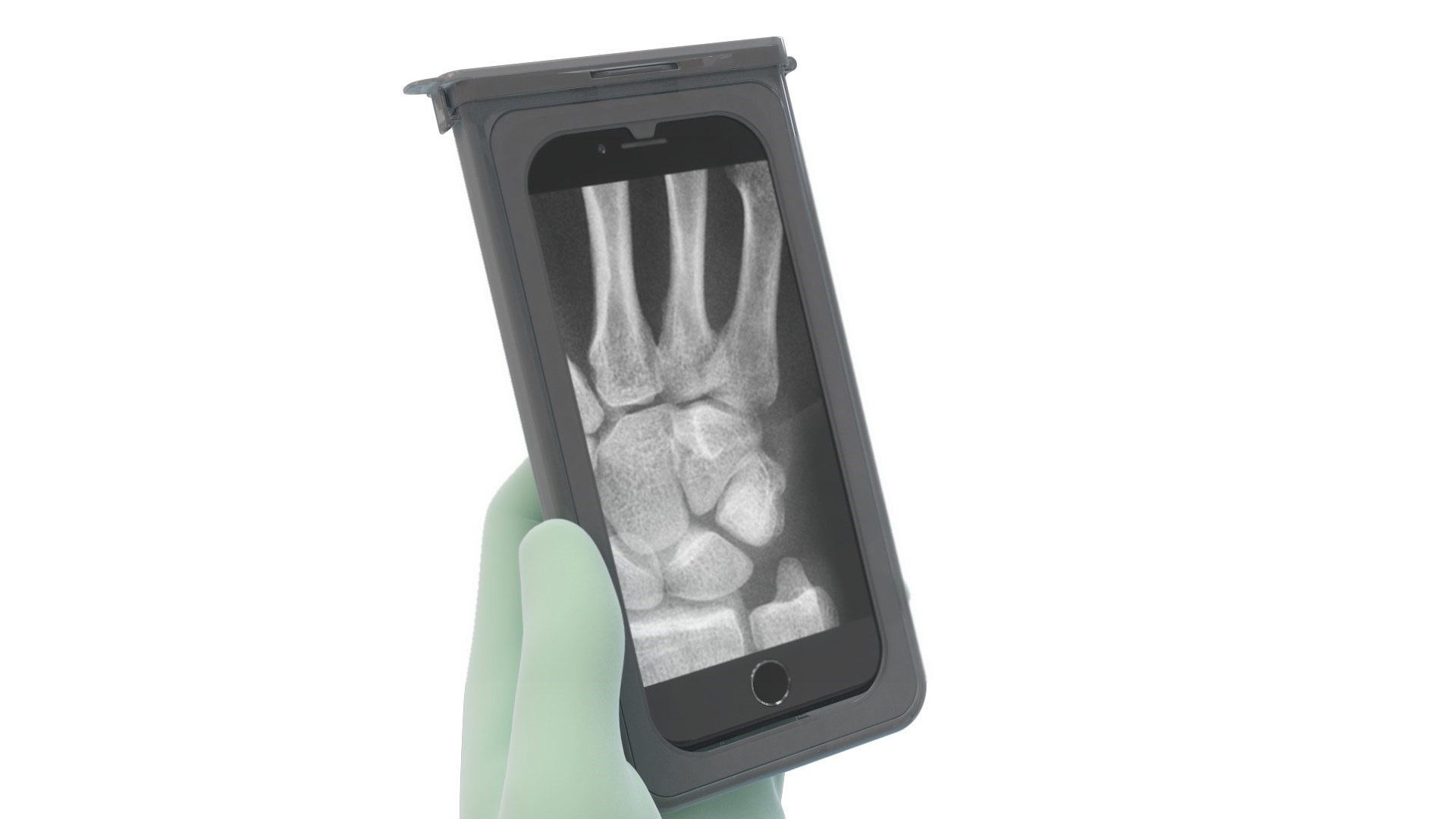
A medical student in the Michigan State University College of Osteopathic Medicine has announced the public launch and availability of the CleanCase sterile mobile devices cover, the first device-specific, fully FDA-complaint product on the market that allows surgeons to safely bring mobile devices into the sterile surgical field. The CleanCase cover, which was developed with investment from Red Cedar Ventures and Quantum Medical Concepts, uses patented technology to allow surgeons full mobile device functionality without compromising patient safety.
Benefits of Brining Mobile Devices into Surgical Field
Surgeons bring mobile devices into the surgical field for two main reasons: to capture media for medical records and to use mobile medical applications. Zondervan, a medical student at Michigan State University College of Osteopathic Medicine, came up with the idea when assisting during an operation. He has over 10 years of clinical and academic medical experience. He serves as the Spartan Innovations Senior Fellow, providing medical advice to startup companies.
CleanCase Sterile Mobile Device Cover Overview
The CleanCase sterile mobile device cover is built on a combination of established operating room techniques and novel technologies that uses a removable sleeve through which the device is passed. Once the mobile device is secured in the device-specific case, the sleeve is removed, and the case is closed and ready to use. The case is made of rigid plastic which features a touchscreen face and an unobstructed camera window, making the mobile device fully functional, protected and easy to manipulate, especially through gloved hands.
“The CleanCase is a first-of-its-kind product that will empower physicians to use the best and latest medical technology on their mobile devices, without ever compromising patient safety,” said Rob Zondervan, Ph.D., Chief Executive Officer at SteriDev, LLC, the Lansing-based medical device company that developed the technology. “Health care mobile applications are developing at a remarkable rate, and in the hands of a great physician, they can make a real difference in the care of patients. Currently, mobile applications are improving the delivery of health care in non-sterile settings. The CleanCase mobile device covers expand their usage into sterile environments so surgical patients can also benefit.”
We are so proud of SteriDev’s recent momentum. Today, we celebrate this milestone achievement,” said Jeff Wesley, executive director, Red Cedar Ventures. “From the company’s launch to the development of its groundbreaking product to FDA approval of CleanCase, Rob and his team are taking SteriDev to the next level.”
Launched in 2016, Red Cedar Ventures has deployed more than $3.5M in pre-seed and follow-on capital into MSU-based startups and licensed technologies. Those dollars have gone on to be leveraged, raising over $100M in outside venture capital.
“Startups are challenging,” explained Wesley, “I admire anyone with the grit and determination to take an idea and forge it into a reality. Watching Rob balance the challenges of a startup with the rigors of a medical residency—that’s a feat. We’re honored to invest in this Spartan startup team.”
