Flow, a London-based medical device startup using a brain stimulation headset and therapy app to treat depression has raised $1.5 million in seed funding led by Khosla Ventures. In Europe, Flow has been approved as a Class II medical device intended for use as a treatment for depression – and is the first approved treatment of its kind in Europe available to buy and use at home. The investment will be used to support Flow’s European rollout, introduce Flow to healthcare clinics, and fund
Read More
FDA| Healthcare FDA Regulation | Policy, News, Analysis, Insights - HIT Consultant
StartUp Health Adds 6 New Healthcare Transformers to its Portfolio
StartUp Health, a platform where health transformers, partners, customers, investors, patients, and caregivers connect and accelerate the progress of the health moonshots announced it has added six new companies to its army of Healthcare Transformers.Every quarter, StartUp Health coaches its army of Health Transformers on how to create value, amplify their story, and ultimately achieve their Health Moonshot. Each company is supported for the entire journey of their business, through multiple
Read More
5G in Healthcare: 7 Advantages & Disadvantages for Providers to Know
It’s a fact. The more bandwidth-intensive connected medical devices and mobile devices our hospitals deploy, the more we are straining our health IT infrastructures. Something has to give. Many communications leaders see 5G technology’s real-time high bandwidth and lower latency access as powerful new technology features that are needed to expand healthcare applications’ capabilities and the functioning of medical devices, robotics, and mobile devices. Some say 5G will be transformative. Others
Read More
Yale, Mayo Clinic, Biofourmis Launches Research to Detect Heart Failure Using Wearable Biosensor Data
Biofourmis has entered a research partnership with the Yale University-Mayo Clinic Center of Excellence in Regulatory Science and Innovation (CERSI). Biofourmis' mobile platform BiovitalsHFTM will be leveraged in a study of patients with heart failure to monitor functional capacity and quality of life to see if greater emphasis should be placed on these measures in the drug approval process.Impact of Heart FailureHeart failure afflicts approximately 6.5 million patients in the United States and
Read More
Notable Nabs $40M to Expand Drug Discovery Platform for Personalized Oncology
Notable, a San Francisco, CA-based company redefining cancer treatment with a clinically validated platform that rapidly advances cancer drug development has raised $40M in Series B funding. The Series B round is co-led by B Capital Group and LifeForce Capital. LifeForce is a returning investor, joined by new investors B Capital and Industry Ventures. This latest round brings Notable’s total funding to over $55M.Notable was founded in 2017 by Matt De Silva after helping his own father Marc, who
Read More
Procyrion Raises $30M to Support Clinical Trials of its Percutaneous Blood Pump Device
Procyrion, Inc., a Houston-based medical device firm developing the first catheter-deployed, intra-aortic pump initially for use in cardiorenal syndrome patients announced it has raised $30 million in Series D funding led by Bluebird Ventures. The round also includes participation from existing investors Fannin Partners, Scientific Health Development, the State of Texas, and an undisclosed strategic investor.The company is developing its AortixTM system, a percutaneous blood pump initially
Read More
What’s the Difference: A Look at Consumer and Medical-Grade Wearables in Healthcare
Can both consumer and medical-grade wearables work together to fill healthcare’s care gaps? Dr. Sunil Kapur shares his insights.
What does Abbott’s Confirm Rx insertable cardiac monitor (ICM) and the latest Apple Watch have in common? The answer: more than you think, and perhaps not nearly enough. Today, consumer and medical-grade wearables may live in different markets, but they are aligning on a similar long-term objective—capturing critical data to improve outcomes.
“People want to
Read More
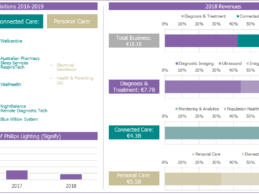
Analysis: Philips’ Evolution to a Pure-play Healthcare Technology Vendor
The last two-to-three years we have seen Philips complete its transformation into a pure-play healthcare technology vendor. It has reduced its interest in its remaining non-healthcare business Philips Lighting/Signify (now owning only a 16.5% share), made 16 healthcare technology acquisitions since the start of 2017 and organized its internal business units into three core healthcare technology segments, Personal Health, Diagnosis & Treatment and Connected Care. A few weeks ago, several of
Read More
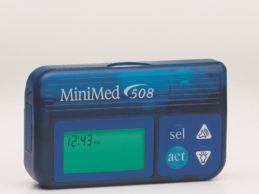
FDA Recalls Medtronic MiniMed Insulin Pumps for Potential Cybersecurity Risks
The U.S. Food and Drug Administration (FDA) is recalling certain Medtronic MiniMed insulin pumps due to potential cybersecurity risks and recommends that patients using these models switch their insulin pump to models that are better equipped to protect against these potential risks. To date, the FDA is not aware of any confirmed reports of patient harm related to these potential cybersecurity risks.Medtronic's MiniMed Insulin PumpsThe FDA is concerned that, due to cybersecurity vulnerabilities
Read More
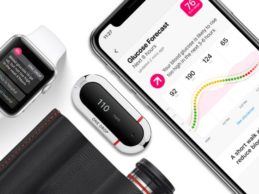
Apple to Sell One Drop’s Blood Glucose Monitors in Select Retail Stores
One Drop, a digital blood glucose monitoring kit for diabetes management will now be available in select retail Apple stores, CNBC reports. Currently sold online in the Apple Store for $69.95, the digital diabetes kit includes a Bluetooth glucose meter, test strips, lancets (optional), and one year of free coaching from a certified diabetes coach.One Drop Blood Glucose MonitorOne Drop’s blood glucose meter ensures clinically proven accurate, reliable results in just five seconds. The meter then
Read More