What You Should Know:
- Medidata, a Dassault Systèmes company and Parexel, a global clinical research organization (CRO) focused on development and delivery of innovative new therapies to advance patient health extends their 15-year global strategic partnership. This extension includes gaining access to Medidata’s continued innovations in clinical trial technology solutions with its unified platform, support of decentralized clinical trials (DCTs), the myMedidata patient portal, Sensor
Read More
Decentralized Clinical Trials
Philips Launches First At-Home, 12-Lead ECG Integrated Solution for Decentralized Clinical Trials
What You Should Know:
- Philips introduced the industry’s first full-service, at-home, 12-lead electrocardiogram (ECG) solution for use in decentralized clinical trials.
- The solution is the most advanced patient-centric ECG offering within the company’s cardiac monitoring portfolio, pairing data readings comparable to clinical, site-based ECGs with Philips leading cloud-based data collection and analysis services.
Patient Attrition Issues
Patient attrition is one of the biggest
Read More
Platform Launches to Help Eliminate Biases in Decentralized Trials
What You Should Know:
- Obviohealth, a decentralized clinical trial platform, has launched a new AI-assisted clinical raters platform to eliminate biases from trials.
The platform Augmented ePRO (electronic patient-reported outcomes), has been designed to assist clinical raters to better score patient outcomes, without biases.
Why It Matters
As more trials take place virtually or decentralized, clinical researchers are at the mercy of the quality of the information provided by the
Read More
Topography Health Launches out of Stealth with $27.5M to Empower Community Physicians to Run Clinical Drug Trials
What You Should Know:
- Topography Health, a clinical trials startup focused on community physicians, today launched out of stealth with $27.5M in funding from Andreessen Horowitz and Bain Capital Ventures.
- Co-founded by former Gerson Lehrman Group CEO Alexander Saint-Amand, Topography is making it possible for your family doctor to offer clinical drug trials as part of their practice. Not just for potentially terminal illnesses, but also for moderate chronic illnesses, where the
Read More
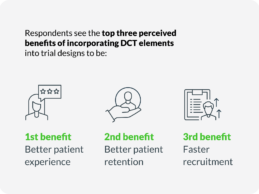
Decentralized Clinical Trials Can Achieve Net Financial Benefits of 5X to 14X
What You Should Know:
- New data from Tufts and Medable reveal on average, decentralized clinical trials (DCTs) are associated with reduced clinical trial timelines and can achieve net financial benefits ranging from five to 14 times for Phase II and Phase III trials, respectively.
- The findings are based on financial modeling and analysis of trial data from the Tufts Center for the Study of Drug Development (CSDD), Tufts University School of Medicine, and more than 150 clinical trials
Read More
77% of Sponsors, CROs Plan to Run Agile Clinical Trials in Next Months
What You Should Know:
- Today Science 37 published a new report suggesting a momentous shift in the way that clinical trials are conducted.
- For the first time, more research sponsors and CROs are planning to run Agile/Hybrid Clinical Trials than ever before. Findings show that the vast majority or 77% of respondents plan to run an agile clinical trial in the next 12 months, compared to 59% for the previous 12 months.
Science 37 Holdings, Inc. (Nasdaq: SNCE) (“Science 37”), a
Read More
Overcoming Key Hurdles in Decentralized Trials with Better Education
As with countless global industries, clinical trials were forced to move key functions online as COVID-19 swept the world in early 2020. Clinical research operations had to rapidly deploy remote approaches that were previously in the planning or pilot stages. While the uptake was rushed, the results have been largely positive. Decentralized clinical trials, and their near cousins that apply a hybrid approach of in-person and remote visits, are increasingly seen as a viable solution for biopharma
Read More
Decentralized Clinical Research Will Be A Paradigm-Shifting Trend
The field of clinical research is on the precipice of change, and for good reason. Traditional clinical trials and processes are primarily confined to sterile, isolated clinical settings that are often narrow in scope and provider-centric. Researchers of tomorrow, however, already are identifying accurate and effective new methods to conduct and draw meaning from their work, after concluding that current models for clinical research are not capturing participants’ real-world, lived experiences
Read More
3 Barriers to Decentralized Clinical Trials Adoption
It’s official. Decentralized clinical trials have disrupted the clinical trial process for good. The COVID-19 pandemic elevated many digital innovations, proving that virtually connecting with patients is not only convenient but necessary when it comes to providing equitable access to cutting-edge treatments and clinical research.
Historically, traditional clinical trials have always experienced recruitment and retention challenges due to inconveniently located trial sites, lack of awareness
Read More
Decentralized Clinical Trials: Keys to Optimizing Diversity and Inclusion
The U.S. pharma industry and research intuitions have long struggled with increasing clinical trial diversity in an effective, sustainable, and scalable fashion. Clinical research, in general, acknowledges the universal struggle of recruiting enough participants from various demographic groups. For example, racial and ethnic minorities have been historically underrepresented in clinical trials—a problem that still persists today.
According to Deloitte, African Americans comprise 13% of the
Read More