We asked several healthcare executives to share their health IT predictions and trends for 2023.
Nate Maslak, the co-founder/CEO of Ribbon Health
Data Personalization: 38% of consumers want more personalized and inclusive healthcare options. In 2023, we’ll see a greater shift towards healthcare enterprises prioritizing this personalization, by innovating their current data infrastructure to show a range of information that lets a patient make an educated care decision based on what
Read More
Clinical Trials | News, Analysis, Insights - HIT Consultant
Real-World Data Startup Crescendo Health Launches with $3.2M
What You Should Know:
- Crescendo Health, a real-world data startup that's drastically reshaping how clinical researchers get access to hard-to-reach patient data to support their clinical research today officially announced its launch with $3.4M in seed funding from Define Ventures as well as the founders and CEOs of many other well-known organizations.
- Founded by the co-founder of Datavant (merged with Ciox in a $7 billion deal), and a top product-management leader from Grand
Read More
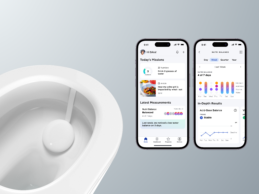
Withings Unveils Mini At-Home Urine Analysis Platform for Clinical Trials & RPM
What You Should Know:
- During a press preview event at CES last night, Withings unveiled, U-Scan, a miniaturized urine health lab that will change the way people and health professionals will monitor vitals from the comfort and privacy of their own bathroom.
- U-Scan consists of a technologically advanced pebble-shaped urine analysis reader that sits within the toilet bowl. Its changeable cartridges are designed to assess specific biomarkers without the need for
Read More
Pangea Biomed Adds $5M for its Multi-Cancer Response Predictor
What You Should Know:
- Pangea Biomed, the biotech company behind ENLIGHT, the multi-cancer response predictor improving the effectiveness of precision oncology, announced $5M in additional funding reaching a total seed round of $12M.
- The latest fundraising is led by angel investor Danny Tocatly and existing investor NFX, and will be used to expand US operations, drive commercial partnerships, and scale product offerings.
Expanding US Operations and Fostering Commercial
Read More
Clinical Trials: 5 Steps to Greater eConsent Adoption
Clinical trials advance much-needed treatments while offering hope to patients and their families. However, the process of enrolling in a study where they will receive investigational medicines, vaccines or procedures can be a source of friction – enough for patients to rethink participation.
The weight of the decision combined with a perceived lack of transparency of information can confuse or dismay even those patients that are most savvy in the medical industry. Even if they do end
Read More
AI/ML Used for Clinical Trial-Site Identification Simultaneously Improves Enrollment Rates and Diversity
Ethnic and racial minorities are commonly underrepresented in clinical trials. This problem is so severe that in April, the U.S. Food and Drug Administration (FDA) expanded upon existing guidance to further emphasize recommendations to sponsors developing treatments to increase enrollment from underrepresented populations in the U.S., including African-American, Hispanic, Asian and other persons of color, in clinical trials. In the updated guidance, the FDA provided details on what sponsors
Read More
How Virtual-First Clinical Trials Scale Access for Participants
Making clinical trials equitable is one of the biggest challenges we face as an industry. We see this time and again, with one of the latest examples coming in the form of a study published in JAMA Network Open that found substantial underrepresentation of Black patients enrolled in pivotal trials for CAR-T therapy.
Women are another demographic negatively affected by inequality in clinical research. Earlier this month [May 2022] the American Heart Association issued a
Read More
AI Improving the Patient Experience of Cancer Care
For cancer patients and their loved ones, days, weeks, and months matter. In fact, anyone who has waited anxiously for test results or a treatment regimen to begin can attest that hours, or even minutes, will drag on with emotional heaviness. From a clinical standpoint as well, time to treatment significantly impacts outcomes. All suspected cancers should be ruled out or confirmed with a diagnosis so treatment can begin as soon as possible. Similarly, patients with incidental findings—masses or
Read More
Why Preventive Healthcare Hinges on Diagnostics Innovation
There is a real sense that we are on the cusp of a diagnostics revolution. This has been spurred on by the pandemic, which at once underscored the tremendous power of mass testing as it became the fundamental basis for decision-making, from our own personal health to national policy. It also revealed gaps in terms of needing to drastically scale up testing capacity and invest in new and innovative digital diagnostics tools.
Innovation in diagnostics, however, is
Read More
Site Enablement Platforms Can Accelerate Clinical Trials by 6 Weeks and Reduce Costs by Over $1M
What You Should Know:
- Florence HealthcareTM, a clinical research technology company headquartered in Atlanta, Georgia, recently announced the completion of a year-long third-party study on the impact of Site Enablement PlatformsTM on clinical research timelines and costs.
- The study by Marketcap Consulting is the first to compare how traditional site management approaches differ from site-first Site Enablement Platforms. The study looked at the impact of traditional sponsor
Read More