What You Should Know:
- New study out from Propeller and Chicago's NorthShore
University HealthSystem shows that asthma patients maintain higher medication
adherence and decrease their rescue inhaler use when using a digital health
platform.
- The study looked at 100 patients recruited from
NorthShore practices, half of whom used Propeller to manage their condition and
half of whom did not.
- The treatment group maintained their high medication adherence at 68%, while the control
Read More
asthma health app
Apple Quietly Acquires Childhood Asthma Monitoring Startup Tueo Health
Apple quietly acquired Tueo Health, a digital health startup developing a solution to assist parents in monitoring asthma-related symptoms in sleeping children, reports CNBC. The exact date of the acquisition is not known; however, Tueo's CEO and COO, Brownwyn Harris and Anura Patil, changed their employers from Tueo to Apple on LinkedIn in late 2018.
Sleep Sensors for Data-Driven Asthma Management
About 10% of children between 5-17 years of age, or more than 7 million kids in the US
Read More
M&A Analysis: 10 Reasons Why ResMed Acquired Propeller Health for $225M
On December 3rd, ResMed announced the acquisition of digital therapeutics maker Propeller Health for $225 million to help establish itself as a leader in COPD management across all stages of the disease. Digital medicines can help to personalize treatments, monitor patients in real-time, detect day-to-day changes in disease condition and increase patient adherence. Founded in 2010, Propeller helps people and their doctors better manage their COPD and asthma. Propeller’s digital medicine platform
Read More
ResMed to Acquire Propeller Health for $225M, Will Operate as Standalone Business
ResMed to acquire digital therapeutics maker Propeller Health for $225 million, establishing itself as a leader in COPD management.ResMed, a provider of cloud-connected medical devices and out-of-hospital software-as-a-service (SaaS) business solutions, today announced it has entered a definitive agreement to acquire digital therapeutics startup Propeller Health for $225M. For ResMed, the acquisition will help establish the company as a leader in COPD management across all stages of the
Read More
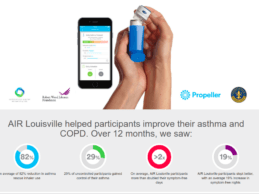
Public Health Study Reduces Asthma Rescue Inhaler Use by 78% in Louisville
Propeller Health and peer-reviewed healthcare journal, Health Affairs, published results from a cross-sector partnership in Louisville, KY, that it has successfully reduced the burden of asthma. As one of the largest public health studies on asthma, Air Louisville is a collaboration among 25 public, private and philanthropic organizations to use digital health technology to improve asthma. The Robert Wood Johnson Foundation (RWJF) funded the successful study, with additional funding from the
Read More
Propeller Health, Express Scripts Partner to Offer Innovative Digital Respiratory Care
Propeller Health and Express Scripts has announced a strategic partnership to provide Propeller's FDA-cleared digital solution to Express Scripts members using inhaler sensors and a mobile app to manage asthma or COPD. This collaboration represents the largest respiratory digital health deployment with a pharmacy benefit manager to date.Propeller's digital sensors enable remote monitoring for patients enrolled in Express Scripts' Pulmonary Care Value Program (PCV), which combines specialized
Read More
Propeller Health Unveils API to Provide Local Asthma Conditions Nationwide
Propeller Health, a digital health company developing solutions respiratory medicine has released the first ever API to provide local asthma conditions to anyone across the United States. The new service, Air by Propeller, is open and free to use that enables other individuals and organizations to use its local asthma condition calculations to improve the health of people with chronic respiratory disease.Propeller has trained a machine learning model based on millions of days of anonymized data
Read More
Propeller Health Lands FDA 510(k) Clearance to Market Sensor for the Ellipta Inhaler
Propeller Health, a Madison, Wisconsin-based provider of digital solutions for respiratory medicine has received FDA 510(k) clearance to market its digital sensor for use with GSK’s Ellipta® patented, dry powder inhaler. The custom sensor designed exclusively for the Ellipta inhaler was cleared as part of a R&D collaboration between Propeller and GSK announced in late 2015.For the R&D collaboration, the Propeller sensor automatically collected and recorded data on the Ellipta inhaler's
Read More
Propeller, Health Vectura Partner To Develop Connected Dry-Powder Inhaler
Digital health company Propeller Health is teaming up with respiratory pharmaceutical company Vectura to develop digitally connected dry powder inhaler (“DPI”) technology inhalers integrated with Propeller's FDA-cleared digital health platform. The partnership will bring a new connected dry-powder inhaler device onto the Propeller platform. Vectura provides a range of pre-metered foil blister-based dry powder inhalers developed to meet patients’ needs in inhalation therapy. The initial focus of
Read More
Propeller Health, Aptar Partner To Develop Connected Metered Dose Inhaler
Digital health company Propeller Health is teaming up with Aptar Pharma (Aptar) to develop the world's first integrated connected metered dose inhaler (cMDI), with an integrated sensor and a novel electronic dose counter. The device is currently available for licensing and is expected to enter clinical studies later this year.
Connected Metered Dose Inhaler Development Details
Pressurized MDIs are the most common type of inhaler device on the market today, with over 600 million units
Read More