- RapidPulse, Inc., a Miami-based medical device company developing a novel aspiration system to treat ischemic stroke, today announced it has raised $15M in Series A funding led by Santé Ventures who was joined by Epidarex Capital, Hatteras Venture Partners, Broadview Ventures, and Syntheon.
- The RapidPulse system was developed by Syntheon, a medical device incubator focused on developing next-generation medical devices, and has commercialized some of the largest and most trusted
Read More
Life Sciences | News, Analysis, Insights - HIT Consultant
Lightship Partners with Castor to Scale Virtual Clinical Trials Worldwide
- Lightship and Castor, providers of decentralized clinical trial solutions, today announced a strategic partnership to run direct-to-patient (hybrid) clinical trials at scale. The partnership combines operational excellence in clinical studies and technology that delivers for the most complex clinical trials.
- Lightship’s end-to-end delivery model combined with an in-house care team provide the best possible patient experience in clinical trials.
- By partnering with Castor’s
Read More
Benchling Launches New RNA Capabilities to Accelerate Drug Discovery
What You Should Know:
- Benchling, the leading life sciences cloud, today announced the launch of RNA Therapeutics R&D, a new digital solution that allows scientists to design, analyze, and develop complex RNA therapeutics in a unified cloud platform for the first time.
- More than 1,000 R&D organizations/customers can now accelerate collaboration, speed, and simplicity for RNA research via Benchling — an important advance for biotech.
Biopharmaceutical R&D teams can
Read More
Medidata Announces Decentralized Clinical Trials Program
What You Should Know:
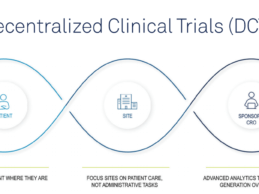
- Today Medidata, a Dassault Systèmes company, announced the launch of the Medidata Decentralized Clinical Trials (DCT) Program, the most comprehensive set of unified, secure technologies that enable full decentralization across the clinical trial continuum.
For the first time ever, drug, vaccine, and medical device developers (sponsors) and contract research organizations (CROs) can take advantage of the only platform offering on the market which combines:
-
Read More
Hawthorne Effect Secures $20M to Decentralize Clinical Trials
What You Should Know:
- Hawthorne Effect, a San Francisco-based solution for decentralizing clinical trials, today announced it has raised $20M in Series A funding led by Northpond Ventures with participation from SignalFire and P5 Health Ventures.
- Founded in 2015, Hawthrone Effect alleviates key issues related to patient recruitment and retention via its tech-driven platform and expansive network of medical professionals.
Hawthrone’s key offerings include:
- Hawthorne Cloud: The
Read More
Medidata Launches New Tech to Transform the Patient Clinical Trial Experience
What You Should Know:
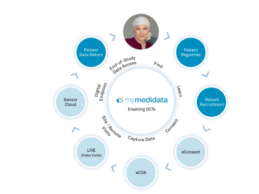
- Today Medidata, a Dassault Systèmes company, launched myMedidata Registries – a new technology that expands and strengthens the company’s myMedidata patient portal to engage patients before and after (i.e., long term follow-up/safety surveillance) a clinical trial.
- The offering, which redefines Medidata’s end-to-end technology solutions and represents a major advance for the life sciences industry, empowers patients to
Read More
HHS Launches VC Partnership to Develop Solutions to Prevent Future Pandemics, Public Health Emergencies
What You Should Know:
- The U.S. Department of Health and Human Services (HHS) launches a new $50M venture capital partnership with nonprofit organization Global Health Investment Corporation (GHIC) to accelerate development and commercialization of technologies and medical products needed to respond to or prevent public health emergencies, such as pandemics, and other health security threats
- Through the BARDA Ventures program, the Biomedical Advanced Research and Development
Read More
PharmStars Launches Pharma-Focused Accelerator for Digital Health Startups
What You Should Know:
PharmStars today announced the launch of its global digital health accelerator to transform how pharma and startups do business. PharmStars is the first and only non-proprietary accelerator for digital health startups that wish to improve their ability to engage with pharma as clients and partners.
For its pharma members, PharmStars offers premium access to a curated group of the most promising digital health startups that align with their digital health strategy. The
Read More
Qualio Secures $50M for Quality Management System Platform for Life Sciences
What You Should Know:
- Qualio — the trusted cloud quality management system software for the entire life sciences ecosystem announced their $50M Series B funding round led by Tiger Global. Menlo Ventures also joined this round, alongside the following current Qualio investors: Frontline Ventures, MHS Capital, Operator Partners, Sorenson Ventures, and Storm Ventures.
- This latest round of funding will support Qualio’s efforts to expand accessibility of their electronic quality management
Read More
PE Firm Thoma Bravo Acquires Clinical Trial Financial Platform Greenphire – Health M&A
What You Should Know:
- Thoma Bravo, a private equity investment firm focused on the software and technology-enabled services sector announced that it has reached an agreement to acquire Greenphire, a financial lifecycle management software for clinical trials, from The Riverside Company. Financial details were not disclosed for the pending transaction.
- The acquisition, which is subject to customary regulatory approvals, is expected to close in the second quarter of this year.
-
Read More