What You Should Know:
- Syapse, a real-world evidence company that informs cancer care, announced a partnership with Illumina aimed at assessing the real-world uptake and actionability of comprehensive genomic profiling in the community oncology health system setting.
- Together, the two companies will work to better understand biomarker testing patterns among clinicians, which have become foundational in identifying treatment options for cancer patients. The insights gleaned from this
Read More
Life Sciences | News, Analysis, Insights - HIT Consultant
COVID-19: As Omicron Variant Spreads, Let’s Not Repeat Rookie Mistakes
No one should be surprised there’s another COVID variant on the loose, and yet seemingly, everyone is. The variant, named Omicron (the fifteenth letter in the Greek alphabet), was first reported to the World Health Organisation (WHO) by South Africa on November 24th. Unlike its name would suggest, Omicron is the seventh COVID-19 variant of concern—but a pandemic-fatigued global public could be forgiven for thinking it’s the twentieth.
Governments across the developed world have pushed
Read More
Quidel Corporation Acquires Ortho Clinical Diagnostics for $6B
What You Should Know:
- Quidel Corporation (NASDAQ: QDEL) (“Quidel”) and Ortho Clinical Diagnostics Holdings plc (NASDAQ: OCDX) (“Ortho”) jointly announced that they have entered into a definitive agreement in which Quidel will acquire Ortho.
- The acquisition will create a global leader in diagnostics, bringing together two highly complementary portfolios with world-class technologies and platforms spanning high-throughput systems to near-patient and at-home testing.
Financial Terms of
Read More
Northwell Launches Virtual Clinical Trial to Reduce Cardiovascular Disease in Black Community
What You Should Know:
- The Feinstein Institutes for Medical Research, the science arm of Northwell Health, recently received a $150k grant from TD Bank, America's Most Convenient Bank, to launch a new research program and the first clinical trial to reduce cardiovascular disease in the Black community and determine the best behavioral interventions to lower Cardiovascular disease (CVD).
Impact of Cardiovascular Disease in Black Community
Cardiovascular disease (CVD) is a major driver
Read More
How 3D Printing is Revolutionizing Orthotics for Patients and Providers
For the 77% of Americans who suffer from foot pain,1 seeking relief has included a litany of options: medications, exercises, insoles and even surgery to name a few. But relief is often hit-or-miss, and insoles that are customized traditionally include a very time-consuming process for patients. They often require lengthy office visits, multiple fit adjustments and even then, not always offering the right fit.
Fortunately, advancements in technology are changing the game. Software,
Read More
70% of US Oncologists Are Wrong About Clinical Trial Diversity
What You Should Know:
- COTA's study reveals that 70 percent of oncologists believe patient populations enrolled in clinical trials for cancer research are representatively diverse.
COTA, Inc., an oncology real-world data and analytics company, announced insights from a study of practicing oncologists that reveals 83 percent of oncologists surveyed believe real-world data is critical to accelerating the development of potentially life-saving cancer drugs and treatments. The study, which
Read More
AWS and Pfizer Accelerate Drug Development and Clinical Manufacturing
What You Should Know:
Amazon Web Services (AWS) announced it is working with Pfizer to create innovative, cloud-based solutions to accelerate drug development and clinical manufacturing for testing in clinical trials.
Today, Amazon Web Services, Inc. (AWS) announced that it is working with Pfizer to create innovative, cloud-based solutions with the potential to improve how new medicines are developed, manufactured, and distributed for testing in clinical trials. The companies are
Read More
Biotech R&D Platform Benchling Closes $100M at $6B Valuation
What You Should Know:
Biotech R&D platform Benchling raises $100M at a $6B valuation, co-led by Franklin Templeton and Altimeter Capital to expand product development and global footprint, particularly in EMEA.
More than 200,000 scientists at over 600 companies and 7,000 research institutions globally have adopted Benchling's R&D Cloud to make breakthrough discoveries and bring the next generation of medicines, food, and materials to market faster than ever before. The R&D
Read More
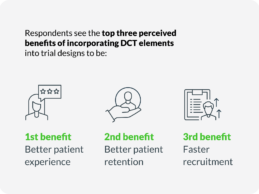
77% of Sponsors, CROs Plan to Run Agile Clinical Trials in Next Months
What You Should Know:
- Today Science 37 published a new report suggesting a momentous shift in the way that clinical trials are conducted.
- For the first time, more research sponsors and CROs are planning to run Agile/Hybrid Clinical Trials than ever before. Findings show that the vast majority or 77% of respondents plan to run an agile clinical trial in the next 12 months, compared to 59% for the previous 12 months.
Science 37 Holdings, Inc. (Nasdaq: SNCE) (“Science 37”), a
Read More
Overcoming Key Hurdles in Decentralized Trials with Better Education
As with countless global industries, clinical trials were forced to move key functions online as COVID-19 swept the world in early 2020. Clinical research operations had to rapidly deploy remote approaches that were previously in the planning or pilot stages. While the uptake was rushed, the results have been largely positive. Decentralized clinical trials, and their near cousins that apply a hybrid approach of in-person and remote visits, are increasingly seen as a viable solution for biopharma
Read More