What You Should Know:
- Walgreens today announced the launch of its clinical trial business to redefine the patient experience and increase access and retention in sponsor-led drug development research.
- Walgreens flexible clinical trial model combines the company’s vast foundation of patient insights, partner-enabled health and technology capabilities and in-person and virtual care options to break through barriers to engaging broader and more diverse communities.
Patient Recruitment
Read More
Life Sciences | News, Analysis, Insights - HIT Consultant
BrightInsight Launches Digital Disease Management Solution for Biopharma
What You Should Know:
- BrightInsight, Inc., provider of the leading global platform for biopharma and medtech regulated digital health solutions launches its Disease Management Solution.
- This configurable suite of digital applications includes a Patient App, Analytics Dashboards and Healthcare Provider Interfaces. The Disease Management Solution, built on the compliant BrightInsight® Platform on Google Cloud, meets biopharma and medtech customers’ core needs out-of-the-box to support
Read More
L7 Informatics Raises $38M to Digitalize Precision Healthcare
What You Should Know:
- L7 Informatics, a leader in software for life sciences that delivers a flexible end-to-end platform for precision healthcare, today announced a $38M investment led by Banneker Partners with participation from its pre-existing investors.
- The company will use the new funding to accelerate product development, expand L7|ESP's global reach, and build on L7's outstanding customer support.
Precision healthcare offers significant improvements in
Read More
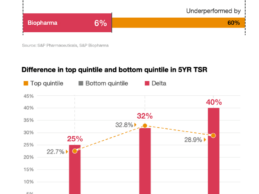
PwC Report: 5 Key Actions for Pharma Companies to Drive Value Growth
What You Should Know:
- A new PwC report offers insights into how pharma companies can translate game-changing science into greater returns.
- Despite many major therapeutic advancements within the last decade, returns from pharma companies lagged the S&P 500 by about one-third and biotech underperformed by 60 percent. The stock performance divide between the front and back of the pack has been widening. PwC’s analysis showed that in 2021, the five-year
Read More
C2i Genomics, Karkinos Healthcare Partner to Bring AI-Powered Cancer R&D to India
What You Should Know:
- C2i Genomics, a cancer intelligence company, announced a strategic partnership with Karkinos Healthcare to develop the first whole-genome sequencing (WGS) MRD test that will further R&D efforts in India.
- This partnership will improve diagnostic labs in India by providing them with the ability to validate and monitor the quality of whole-genome cancer screening and minimal residual disease (MRD) products faster.
AI-Powered Cancer Detection and Monitoring
Read More
Watchmaker Genomics Raises $40M to Expand Clinical Sequencing Product Offerings
What You Should Know:
- Watchmaker Genomics, a life sciences company specializing in the development of high-stringency applications focused on the reading, writing, and editing of DNA and RNA raises $40M in an oversubscribed Series A, bringing total funding to date to $53.5M. The round was led by Decheng Capital, with co-investment from Eclipse Ventures.
- Watchmaker Genomics applies advanced enzymology to enable breakthrough applications for the reading, writing, and
Read More
Outdated Pain Models & The Rise of New Science on Chronic Pain
More than 50 million adults in the United States – more than 20% – suffer from chronic pain, and more than 19.6 million adults have pain that interferes with their lives more days than not. The estimated cost of chronic pain is $635 billion – more than heart disease and cancer combined – due to the number of days missed from work, treatment costs, and other factors. Chronic pain is the leading cause of disability, but the current pain treatment system isn’t working. This is because it hasn’t
Read More
Phenomix Sciences Launches Obesity Biobanking Registry Studying Personalized Treatment Options Based on Genetics
What You Should Know:
- Biotech company Phenomix Sciences launches its biobanking registry, marking the first obesity registry to study the impact of personalized treatment based on phenotyping. The impact of this biobanking registry and the data it will yield has the potential to change the entire system’s approach to obesity, from a treatment recommendation to the way payers determine coverage and how the industry combines diagnostics testing with drug therapy.
- The
Read More
Genetics Startup Pleno Launches Out of Stealth with $15M
What You Should Know:
- Pleno, a multi-omics startup, is emerging out of stealth with a $15M Pre-Series A financing and Greg Lucier as Chairman of the Board of Directors. Founded by Pieter van Rooyen, a well-known biotech engineer who built and sold his last company, Edico Genome, to Illumina for $100M. Edico's Dragen technology has become a staple in genetics research.
- Pleno's Hypercoding technology has the potential to revolutionize biological target detection for
Read More
Reproducibility, Trust, and the Digital Laboratory
Over the last decade, there has been an increasing recognition that results published in scientific journals often cannot be reproduced by other scientists. This has been called the “reproducibility crisis” and has been described in a number of studies. A 2016 Nature article reported that more than 70% of surveyed researchers tried and failed to reproduce another scientist's experiments, and more than half have failed to reproduce their own experiments. A 2021 study reported that fewer than half
Read More