
What You Should Know:
– Wheel and imaware launch the first and only clinician-administered home-based COVID-19 lab testing in the nation, priced at $135.
– A trained healthcare professional will collect the sample at the patient’s home to reduce the risk of false negatives associated with self-swabbing.
– CDC-authorized home-based tests can be delivered the same day, then Wheel clinicians trained in COVID-19 protocols are in contact with patients who test positive to provide immediate care strategies.
– The initial roll-out starts in Texas today, but they’re swiftly moving to offer this service nationwide.
With the need for efficient COVID-19 testing growing increasingly urgent, Wheel and imaware™ have formed a partnership to provide –home-based COVID-19 lab testing within FDA guidelines to patients across Texas, administered by a licensed health professional and supported by telehealth. The companies are combining their areas of expertise to help people at the highest risk or with limited access to testing get screened sooner in order to receive care by specially trained clinicians through telemedicine.
Respiratory Coronavirus Test Now Available Only to Texas Residents
The respiratory coronavirus test is administered by a healthcare professional who performs the sample collection in the home environment, aligning with FDA guidelines on COVID-19 testing. Though tests are currently available only to Texas residents, the partners are working swiftly to make tests accessible nationwide as soon as possible.
The coordinated effort brings together telehealth partners who specialize in different aspects of care delivery, along with a lab that can process tests rapidly and in compliance with CDC and FDA protocols. Wheel provides virtual care clinicians specially trained in CDC-guided COVID-19 care strategies; imaware provides the tech-enabled patient portal and the CDC Emergency Use Authorized (EUA) home-based tests, and national CLIA-certified lab partners will provide access to high-quality laboratory services that can scale up testing operations immediately to meet demand.
The imaware test follows CDC protocols and is administered by a healthcare professional, potentially reducing the risk for false negatives. Self-collected COVID-19 swab tests are potentially at greater risk for inaccurate results, and not currently authorized by the FDA.
How It Works
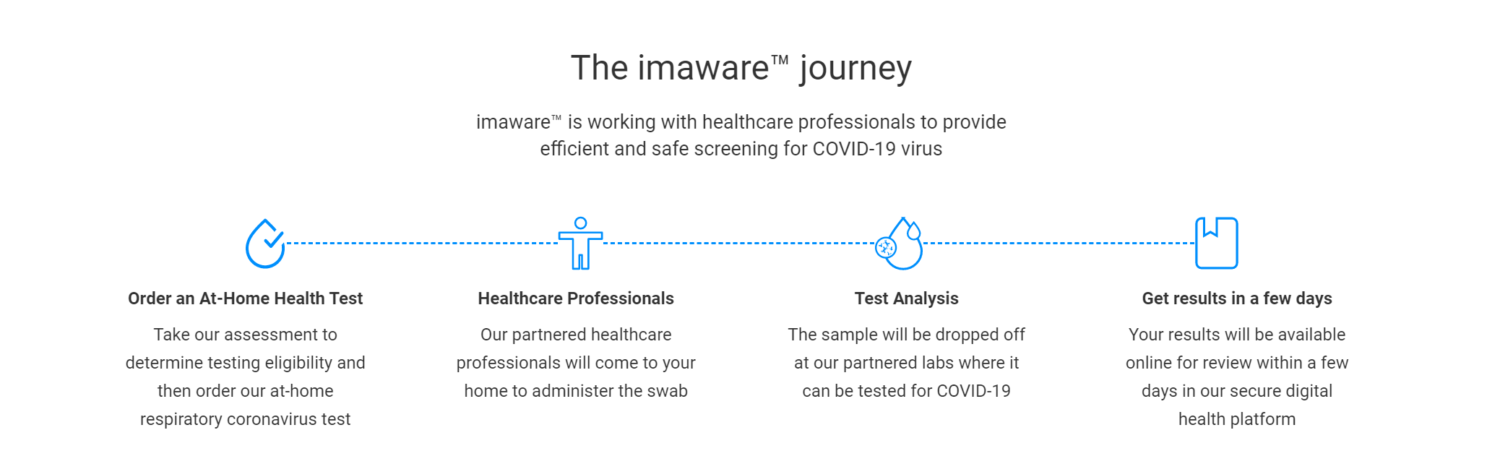
The CDC-approved test, administered in a patient’s home by a licensed clinician and completed by an FDA-authorized laboratory, is accompanied by screening to ensure that those at higher risk or those impacted by a scarcity of testing resources receive the tests in accordance with rapidly changing protocols. The process begins with a 10-question respiratory coronavirus screening and risk assessment via the imaware patient portal, which determines whether the patient is deemed eligible for testing. For patients that qualify for the test, the Wheel clinician orders an imaware test to be delivered to their home within 24 hours, and in some cases same-day. After a medical professional collects the sample, patient results are reviewed and a Wheel clinician provides ongoing remote care for those testing positives for COVID-19, notifying the public health system of the positive result.
“In this evolving health crisis, our highest priority is to ensure that the people at highest risk get the accurate testing and care they need,” said Michelle Davey, CEO of Wheel. “By providing a home-based option for testing that starts with a virtual clinical assessment and then is administered in-home by a registered clinician, we are making more tests available to those who need them most — ensuring that tests are not directed away from areas of greatest need.”
Pricing & Initial Availability of 5k Test Kits Available to Patients in Texas
This week, a controlled roll-out of 5,000 test kits are available to patients in Houston, Texas with plans to expand soon to Austin, San Antonio, and Dallas. In the coming weeks, 10,000 test kits will be available per week, with plans to rapidly scale this number up into 100,000 per week by the end of April nationwide as supplies are made available.
At this time, the cost for the test is $135, which includes access to board-certified clinicians trained by Wheel in COVID-19 protocols and telehealth best practices. imaware™ is in the process of seeking reimbursement on behalf of patients to extend services to the greatest number of people possible.
Patients based in Texas can begin their at-home health assessment at www.imaware.health.
