Medical device design has been going through sweeping
changes over the last decade. Ten years ago,
medical device companies weren't concerned with delivering consumer-level
design: Devices that are both attractive and intuitively easy to use by a wide
variety of users. Then the Affordable Care
Act was passed, and adherence and healthy behavior change became a
regulatory requirement.
Our firm, which has been a long-time proponent of the
“consumerization” of medical
Read More
FDA| Healthcare FDA Regulation | Policy, News, Analysis, Insights - HIT Consultant
Scanwell Health’s FDA-Cleared, Smartphone-Enabled UTI Test Kit Now Available at Amazon
What You Should Know:
- LA-based Scanwell Health announced today that its at-home UTI test kits - featuring the first FDA-cleared urinalysis app that lets people test for a UTI without having to visit a lab or doctor - is now available on Amazon.com.
- The kits are priced at 3 for $15. Happy to send a graphic or answer any questions you have. Until now, the kits have been available only directly through the Scanwell Health web site.
- Scanwell enables clinical-grade testing, instant
Read More
Freespira Secures $10M for FDA-Cleared Digital Therapeutic to Eliminate Panic Attacks, PTSD Symptoms
What You Should Know:
- Lightspeed Venture Partners, the VC behind Nest and GrubHub, is leading a $10 million round for Freespira, an FDA-cleared digital therapeutic proven to significantly reduce or eliminate panic attacks and PTSD symptoms by training users to normalize respiratory irregularities.
- In 28 days, Freespira can reduce or eliminate panic
attacks and PTSD symptoms from home with just a tablet, sensor, and custom app.
There’s no medicine with possible side effects and no need
Read More
Pear Therapeutics Raises $80M to Advance Prescription Digital Therapeutics
What You Should Know:
- Pear Therapeutics today announced that it has
successfully closed an $80 million Series D financing led by SoftBank Vision
Fund 2.
- Pear is the
leader in prescription digital therapeutics and the first company to receive
FDA authorization for a prescription digital therapeutic (PDT) to treat
disease.
- Pear currently has three FDA authorized therapies, reSET, reSET-O and Somryst, for substance use disorder, opioid use disorder, and chronic insomnia,
Read More
Everlywell Secures $175M to Expand Position in At-Home Testing Market
What You Should Know:
- Everlywell secures $175M in Series D funding to expand
its virtual care offerings, scale its testing and infrastructure, drive
clinical research and disease management through testing, and grow its national
leadership position in the at-home testing market.
- Since launching in 2016, more than one million people have used Everlywell's tests and digital platform to manage their health and wellness. As more people turn toward digital platforms for their health
Read More
Brigham, Biofourmis Co-Develops Tech Solution to Enable Home Hospital Care
What You Should Know:
- Brigham is working with Biofourmis on a national
rollout of their co-developed technology solution, which has been refined and
scaled for use by other healthcare systems across the country seeking to
establish and execute similar home hospital programs to alleviate COVID-related
capacity issues and to receive 1:1 parity on payments from CMS.
- The Hospital@HomeTM platform provides a turnkey,
end-to-end ecosystem for any health system that wants to quickly launch
Read More
Recent Executive Hires: CVS Health New President, Cleveland Clinic/Amwell Joint Venture Leadership, Others
CVS Health Corporation names Neela Montgomery Executive Vice President and President of CVS Pharmacy/Retail, effective November 30, 2020. Montgomery will oversee the company's 10,000 pharmacies across the United States. Montgomery, currently a Board Partner at venture capital firm Greycroft, most recently served as chief executive officer of furniture retailer Crate & Barrel and has nearly 20 years of global retail experience.
The Cleveland Clinic and Amwell joint venture appoint
Read More
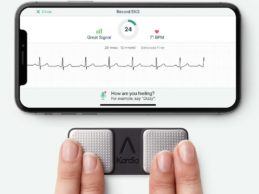
AliveCor Receives FDA Clearance of Next-Gen EKG Algorithms
What You Should Know:
- AliveCor announced they received FDA clearance of new
algorithms for use with their personal EKG devices, KardiaMobile and
KardiaMobile 6L. These additional determinations will be available via a
software upgrade for the Kardia devices in 2021.
- The additional FDA-cleared algorithms double the number
of heart rhythm disturbances that AliveCor’s Kardia devices can detect,
broadening the number of patients who are able to use their remote
Read More
White House Coronavrius Task Force Doubles Down on Rapid Testing Strategy
What You Should Know:
- White house coronavirus task force doubles down on rapid testing strategy to fight the coronavirus as some states say they don’t have the supplies to comply with the federal government’s advice.
- This article was originally published by the Center for Public Integrity, a nonprofit investigative news organization based in Washington, D.C.
The White House coronavirus task force is doubling down on part of its strategy for halting the spread of the virus:
Read More
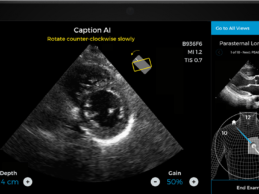
Gates Foundation Awards Caption Health $4.95M Grant to Develop AI-Guided Lung Ultrasound System
What You Should Know:
- Bill & Melinda Gates Foundation awards Caption
Health a $4.5M grant to support the development of an AI-guided lung ultrasound
system.
- The grant from the Bill & Melinda Gates Foundation
will be leveraged to create new AI technology that allows medical professionals
without prior ultrasound experience to perform lung ultrasounds, expanding
access to quality medical care.
Caption Health, a leading medical
artificial intelligence (AI) company, today
Read More