What You Should Know:
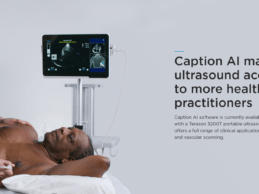
- Caption Health raises $53 million series B round to
fuel commercialization and expansion of its FDA-approved AI ultrasound
technology. This is on the heels of its receiving FDA clearance, designated as
a breakthrough technology to fight against COVID-19.
- As ultrasounds, especially cardiac ultrasounds, have
become increasingly important during a time of COVID when resources are
stretched, using AI to help more healthcare professionals take high-quality
images is
Read More
FDA Cleared Devices
Heart Failure is Detectable at Point of Care Using ECG-Enabled Stethoscope, Mayo Clinic and Eko Finds
- Eko and Mayo Clinic announced they have proven that heart failure is detectable during routine physical exams, further validating the Eko DUO digital stethoscopes as a heart failure screening tool. - Today’s news marks the first time that a point of care device with a single lead ECG combined with an AI algorithm identified low ejection fraction in patients.- The results of these findings, which are comparable to research published earlier this year in NatureMedicine, were presented over the
Read More
FDA Clears AI-Powered EchoGo Core for Early Detection of Cardiovascular Disease
- Ultromics has just become one of the first companies to have 510(k) FDA clearance for an AI medical device. - EchoGo will now be available for clinicians in the UK and US to use to help them identify cardiovascular disease earlier. - EchoGo automates cardiac measurements and is the first AI application to measure cardiac strain, improving patient care and outcomes.Ultromics, the UK-based health technology firm at the forefront of applying artificial intelligence to echocardiography has
Read More
Biofourmis Analytics Engine Receives FDA Clearance for Ambulatory Physiologic Monitoring
- Biofourmis received 510(k) clearance from the FDA for its Analytics Engine as a medical device for ambulatory physiological monitoring.- Milestone approval establishes an AI-powered solution as the foundation for future disease-specific predictive management tools from Biofourmis.- This FDA approval is the second market authorization for Biofourmis, having earned the agency's approval in May 2019 for its Biovitals RhythmAnalytics platform.Boston-based Biofourmis has received 510(K) clearance
Read More
Healthy.io Nabs $60M for FDA-cleared Smartphone-Based Urinalysis for Chronic Kidney Disease
- Healthy.io raises $60M led by Corner Ventures for its FDA 510(k) cleared smartphone-based ACR test to be used in the aid of diagnosing chronic kidney disease (CKD).
- The funding round will be used to accelerate Healthy.io’s global expansion and product development.
- Healthy.io’s solution allows immediate electronic medical record (EMR) connectivity through the automated smartphone scan.
Healthy.io, the fast-growing Israeli digital health company that has turned
the
Read More
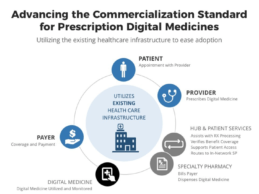
Cognoa, EVERSANA Partner to Advance the Commercialization Standard for Prescription Digital Medicines
- Cognoa and EVERSANA form a partnership that will enable prescription, dispensation, and reimbursement of Cognoa’s behavioral health products.
- Cognoa is developing digital medicine solutions to change the standard of care in pediatric behavioral health to improve the lifelong outcomes for children.
- Cognoa selected EVERSANA, a fully integrated and independent commercial services platform, to develop and manage a go-to-market strategy that ensures comprehensive market
Read More
OptiScan Biomedical Nabs $20M for FDA-Cleared Continuous Glucose Bedside Monitor for ICU
OptiScan Biomedical, a developer of an innovative continuous monitoring system for use in the surgical intensive care unit (SICU) has raised $20 million in Series E funding. The company plans to use the funding to support the commercialization of the company’s lead product, the OptiScanner 5000, in the United States. OptiScan will also use a portion of the funding to continue the expansion of the company’s OptiScanner platform to monitor additional analytes of interest for critically ill
Read More
Why the FDA Is Doing an Overhaul of the Medical Device Approval Process
Since 1976, the U.S. Food and Drug Administration (FDA) has allowed manufacturers to apply for an accelerated pathway to bringing their devices to the market. It's called the 501(k) Premarket Notification. In short, if a new device gets proved safe and effective to a device that's already available, the new device can get cleared because it's considered substantially similar to the older one. The FDA also allows for the first-cleared device (also known as the predicate) to be another product
Read More
University Hospitals Adopts Needle-Free Blood Draw Technology
University Hospitals Cleveland Medical Center adopts needle-free blood draw technology to reduce anxiety and risks for hospitalized patients.University Hospitals (UH) Cleveland Medical Center in Ohio, announced that it has implemented the PIVO needle-free blood draw device from San Francisco-based medical device innovator Velano Vascular at UH Cleveland Medical Center for inpatient blood draws. UH is the first health system in Ohio to deliver this enhanced experience to patients and is helping
Read More
BioTel Care Unveils Next-Gen Wireless Blood Glucose Monitor for Diabetes Management
BioTel CareTM (formally known as Telcare), a BioTelemetry company has announced the release of its next-generation wireless blood glucose monitor for diabetes management. BioTel Care developed the first FDA-cleared, cellular-enabled glucometer which supports real-time transmission and consolidation of patient data in an FDA-cleared cloud. Building on the success of this technology, BioTel Care is launching its next-generation blood glucose monitor, which includes an innovative touch-screen user
Read More