What You Should Know:
- Valencell, the company whose optical heart rate technology enabled the ability of wearables to accurately measure cardiovascular vitals during exercise, today announced plans to launch its own branded product line in the digital health sector as it concentrates efforts to bring solutions to market to manage chronic diseases.
- The company’s first product candidate being showcased at CES is focused on helping people monitor and manage hypertension by combining
Read More
FDA Clearance
Dexcom Receives FDA Clearance for G7 Continuous Glucose Monitoring (CGM) System
What You Should Know:
- The FDA has cleared Dexcom G7 continuous glucose monitoring (CGM) system in the U.S. for people with all types of diabetes ages two years and older, giving more people than ever access to a powerfully simple diabetes management solution.
- Cleared as an integrated continuous glucose monitoring (iCGM) system, Dexcom G7 will be part of the most connected CGM ecosystem in the world, with real-time connectivity that can drive integrated insulin delivery systems, connect
Read More
Eyenuk Raises $26M for AI-Powered Eye Screening & Predictive Biomarkers
What You Should Know:
- Eyenuk, Inc., a global artificial intelligence (AI) digital health company and the leader in real-world applications for AI Eye Screening™ and AI Predictive Biomarkers™, today announced it has secured $26 million in a Series A financing round, bringing the Company’s total funding to over $43 million.
- The capital raise was led by AXA IM Alts and was joined by new and existing investors including T&W Medical A/S, A&C Foelsgaard Alternativer ApS, Kendall
Read More
Optellum Raises $14M for AI-Enabled Lung Cancer Diagnosis
What You Should Know:
- Optellum, an Oxford-based digital health company that provides a breakthrough AI platform to diagnose and treat early-stage lung cancer raises $14M in Series A funding led by Mercia, with additional investors Intuitive Ventures and Black Opal Ventures. Existing investors, including St John's College in the University of Oxford, IQ Capital, and the family office of Sir Martin & Lady Audrey Wood, also participated in this round.
- The investment will enable
Read More
Assisted Polyp Detection Device Receives FDA Clearance
What You Should Know:
- Iterative Scopes, a pioneer in precision medicine technologies for gastroenterology, and Provation, the premier software and SaaS provider of clinical productivity and workflow automation solutions, announced recently that SKOUT™ has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for adults undergoing colorectal cancer screening or surveillance.
- The device was evaluated in the largest U.S.-based multicenter clinical study for a
Read More
Eko Lands $2.7M NIH Grant to Train Pulmonary Hypertension AI
What You Should Know:
- Eko, a digital health company applying machine learning in the fight against heart and lung disease, today announced that it was awarded a $2.7M Small Business Innovation Research (SBIR) Direct Phase II grant by the National Institutes of Health’s (NIH) Department of Health and Human Services (HHS).
- The grant will fund the development of a machine learning algorithm that detects and stratifies pulmonary hypertension (PH) using phonocardiogram (PCG) and
Read More
RapidAI Awarded FDA Clearance for AI-Powered CT Scans to Identify Intracerebral Hemorrhage
What You Should Know:
- RapidAI, the global leader in neurovascular and vascular AI-enhanced clinical decision support and patient workflow, today announced it has received FDA clearance for Rapid Hyperdensity, the newest addition to the RapidAI platform.
- The tool empowers physicians to quickly assess the severity of injury in patients with acute neuro conditions such as traumatic brain injury and brain hemorrhages – allowing for better and faster patient care decisions.
AI Powered
Read More
FDA Clears Eko’s Heart Disease Detection AI for Adults and Pediatrics
What You Should Know:
- Today, Eko, a digital health company advancing heart and lung disease detection, has announced the FDA has issued clearance for its Eko Murmur Analysis Software (EMAS), the first and only machine learning algorithm to screen for valvular heart disease (VHD).
- The next generation of Eko’s murmur detection capabilities grants Eko the first and only smart stethoscope on the market that can identify and
Read More
Fitbit Receives FDA Clearance for New PPG Algorithm to Identify AFib
What You Should Know:
- Fitbit announced it has received clearance from the FDA for its PPG (photoplethysmography) algorithm to identify atrial fibrillation (AFib), a condition that affects more than 33.5 million people globally.
- The algorithm will soon be available to users in the U.S. through Fitbit’s new Irregular Heart Rhythm Notifications feature on eligible heart-rate tracking devices.
Read More
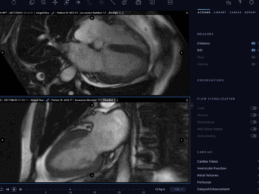
Arterys Receives FDA Clearance for Next-Gen Cardiac MRI App
What You Should Know:
- Arterys, the world's leading vendor-neutral artificial intelligence (AI) platform, has announced several new modules to its already robust Cardio AI clinical application and an additional FDA AI clearance based on deep learning.
- Cardio AI is a comprehensive, cloud-based post-processing solution that leverages AI and deep learning to provide extremely accurate, automated, fast, repeatable analysis of cardiac MRI images.
Cardio AI: AI-Assisted Cardiac MRI
Read More