What You Should Know:
- Smileyscope™, a pioneer in virtual reality (VR) therapeutic solutions, has received Class II clearance from the U.S. Food and Drug Administration (FDA) for its Smileyscope™ Therapy system.
- The FDA clearance marks Smileyscope as the first and only VR Analgesic™ available in the US.
Digital Therapeutics Leadership
Smileyscope's FDA clearance underscores its leadership in Digital Therapeutics, a category defined as delivering medical interventions directly to
Read More
FDA Clearance
FemTech: Mosie Baby Awarded FDA Clearance for At-Home Intravaginal Insemination
What You Should Know:
Mosie Baby, a pioneering at-home fertility care company, has secured FDA Class II clearance for its Mosie Baby Kit making it the first and only FDA-cleared over-the-counter kit for use in intravaginal insemination (IVI).The kit was created to support those who are unable to conceive with intercourse or for whom intercourse is not an option. Following its FDA 510k Class II clearance, Mosie Baby looks forward to expanding access to its Mosie Baby Kit which was
Read More
RapidAI Receives FDA Clearance for Rapid SDH (Subdural Hematoma) for Trauma Care
What You Should Know:
- RapidAI has received FDA clearance for Rapid SDH, its AI-powered module for the detection and notification of suspected hemispheric acute and chronic subdural hematoma.
- The need for the RapidAI solution is urgent, with SDH cases in US patients expected to increase by nearly 80% before 2040.
AI Module for Detection of Hemispheric Subdural Hematomas
Rapid SDH leverages AI to help neurocritical care teams identify suspected hemispheric subdural hemorrhage
Read More
Empatica Awarded New FDA Clearance for Cardiac Digital Biomarkers
What You Should Know:
- Empatica, a digital health and AI company, has obtained FDA 510(k) clearance for two new digital biomarkers, pulse rate, and respiratory rate, on its Empatica Health Monitoring Platform.
- This clearance expands the platform to include a total of six FDA-cleared digital biomarkers, out of the 128 digital measures supported, making it one of the most comprehensive solutions available for use in clinical trials.
- The Empatica Health Monitoring Platform comprises a
Read More
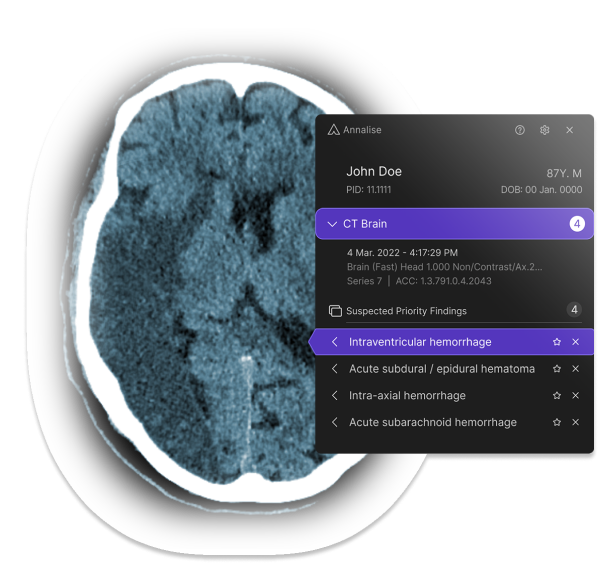
Annalise.ai Receives FDA Clearance for Radiology Triage Device
What You Should Know:
Annalise.ai, a leader in AI-powered medical imaging solutions, announced the receipt of 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the triage and notification of obstructive hydrocephalus (OHCP) on non-contrast brain CT scans.Annalise.ai has also received FDA Breakthrough Device Designation for its obstructive hydrocephalus software tool. This is the first radiology triage device to be granted Breakthrough status since the inception of the
Read More
What Role Can Consumer Health Technology Play in Diagnoses?
There’s an ongoing debate regarding the role that consumer health technology, like wearable health devices (i.e., smartwatches), can play in diagnostics, now and in the future. Because this is a relatively new technology, the scope of its potential impact is, at present, only scraping the surface.
Even so, smartwatches and their connected health apps are reshaping the healthcare industry. This technology has the ability to not only make personalized healthcare more widely accessible, but its
Read More
AI-Enabled Butterfly Network Lung Tool Receives FDA Clearance
What You Should Know:
- Today, Butterfly Network announced it received 510(k) clearance from the FDA for a groundbreaking AI-enabled tool named AI-enabled Auto B-line Counter that will help physicians assess adults’ lungs and accelerate providers’ abilities to make informed treatment decisions. Butterfly used data inputs from hundreds of sites across the country to train and develop its AI algorithms, offering potential for a broad and diverse range of age, gender, body mass index, ethnicity,
Read More
TytoCare Receives FDA Clearance for AI-Powered Tyto Insights for Wheeze Detection
- TytoCare, a virtual care company enabling accessible, high-quality primary care from home, today announced that it received FDA clearance for its Tyto Insights for Wheeze Detection, paving the way for its rollout in the US.
- The wheeze detection algorithm expands the company’s existing AI-powered Tyto Insights™ smart diagnostic capabilities, filling the quality gaps currently experienced with traditional telehealth and alleviating challenges imposed by the ongoing shortage of healthcare
Read More
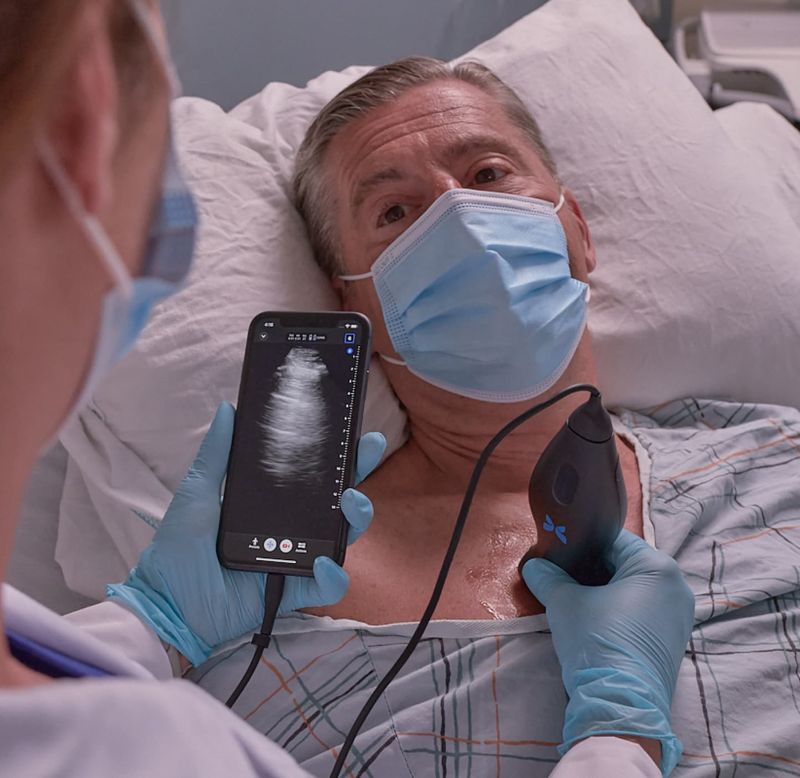
Clarius Awarded FDA Clearance for AI Ultrasound Musculoskeletal Imaging App
What You Should Know:
- Clarius Mobile Health, a leading provider of high-definition handheld ultrasound systems, receives 510(k) clearance for its new MSK AI model, which automatically identifies and measures tendons in the foot, ankle, and knee using artificial intelligence (AI).
- The new MSK AI model will be available soon with the Clarius L7 HD3 and Clarius L15 HD3 high-frequency, wireless handheld ultrasound scanners.
AI Driven Imaging To Improve
Read More
Selux Receives FDA Clearance for its Rapid Antibiotic Susceptibility Test (AST) System
What You Should Know:
- Selux Diagnostics has been granted 510(k) clearance from the US FDA for its Next Generation Phenotyping (NGP) System — a rapid antibiotic susceptibility (AST) testing platform able to determine a bacteria’s susceptibility to a specific panel of antimicrobial agents.
- In vitro antimicrobial resistance test enables clinical labs to deliver targeted therapeutic results days faster than the current standard of care, clearing the path for personalized antibiotic
Read More