- Sight Diagnostics, the company working to provide patients with lab-grade blood testing results in minutes, has received US FDA 510(k) clearance for its finger-prick blood test, OLO analyzer to be used in laboratories run by diagnostic providers and hospitals.
- Sight Diagnostics’ patented OLO analyzer digitizes blood into images to perform the most common blood test in minutes, from just two drops of blood
- The FDA clearance follows clinical trials at Boston Children’s Hospital,
Read More
FDA Clearance
Heart Failure is Detectable at Point of Care Using ECG-Enabled Stethoscope, Mayo Clinic and Eko Finds
- Eko and Mayo Clinic announced they have proven that heart failure is detectable during routine physical exams, further validating the Eko DUO digital stethoscopes as a heart failure screening tool. - Today’s news marks the first time that a point of care device with a single lead ECG combined with an AI algorithm identified low ejection fraction in patients.- The results of these findings, which are comparable to research published earlier this year in NatureMedicine, were presented over the
Read More
FDA Clears AI-Powered EchoGo Core for Early Detection of Cardiovascular Disease
- Ultromics has just become one of the first companies to have 510(k) FDA clearance for an AI medical device. - EchoGo will now be available for clinicians in the UK and US to use to help them identify cardiovascular disease earlier. - EchoGo automates cardiac measurements and is the first AI application to measure cardiac strain, improving patient care and outcomes.Ultromics, the UK-based health technology firm at the forefront of applying artificial intelligence to echocardiography has
Read More
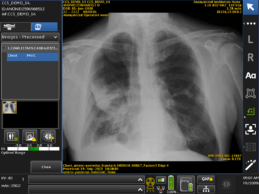
FDA Clears GE Healthcare’s AI Algorithms Embedded on Mobile X-Ray Device
- GE Healthcare awarded Food and Drug Administration’s 510(k) clearance of Critical Care Suite, an industry-first collection of artificial intelligence (AI) algorithms embedded on a mobile X-ray device.
- Algorithms help radiologists prioritize critical cases with a suspected pneumothorax – a type of collapsed lung – by immediately flagging critical cases to radiologists for triage, which could drastically cut the average review time from up to eight hours.
- Critical Care Suite offers
Read More
Healthy.io Nabs $60M for FDA-cleared Smartphone-Based Urinalysis for Chronic Kidney Disease
- Healthy.io raises $60M led by Corner Ventures for its FDA 510(k) cleared smartphone-based ACR test to be used in the aid of diagnosing chronic kidney disease (CKD).
- The funding round will be used to accelerate Healthy.io’s global expansion and product development.
- Healthy.io’s solution allows immediate electronic medical record (EMR) connectivity through the automated smartphone scan.
Healthy.io, the fast-growing Israeli digital health company that has turned
the
Read More
What’s AI’s Holdup in Healthcare? The Answer Is In The Cloud
When you consider what technology has the most potential to transform healthcare over the next decade and beyond, no doubt your first thoughts turn to artificial intelligence (AI), machine learning (ML) and deep learning (DL). Already these technologies are showing promise in helping clinicians work faster and smarter, identifying trends, alerting us to signs that can predict and prevent diseases, and providing insights into potential treatments and cures.
But AI, ML, and DL alone cannot
Read More
What’s the Difference: A Look at Consumer and Medical-Grade Wearables in Healthcare
Can both consumer and medical-grade wearables work together to fill healthcare’s care gaps? Dr. Sunil Kapur shares his insights.
What does Abbott’s Confirm Rx insertable cardiac monitor (ICM) and the latest Apple Watch have in common? The answer: more than you think, and perhaps not nearly enough. Today, consumer and medical-grade wearables may live in different markets, but they are aligning on a similar long-term objective—capturing critical data to improve outcomes.
“People want to
Read More
MaxQ AI Launches FDA Cleared, CE Approved Slice-Level Intracranial Hemorrhage Detection
MaxQ AI, an Israeli-based medical diagnostic AI company has launched the company’s new ACCIPIO Ax solution, which features SliceMap, an integrated view that guides clinicians rapidly to CT slices with suspected intracranial hemorrhage (ICH) without leaving their PACS viewer. Slice-Level Intracranial Hemorrhage DetectionACCIPIO Ax is FDA Cleared, CE Approved, and the second component of MaxQ AI’s ACCIPIO ICH Platform, the complete ICH solution for stroke and head trauma assessment. Radiology
Read More
Neural Analytics Lands $22M for Robotically Assisted Ultrasound System for Brain Health Assessment
Neural Analytics, Inc., a Los Angeles, CA-based medical robotics company developing and commercializing technologies to measure and track brain health has raised $22 million in Series C funding led by Alpha Edison and the Company has also issued warrants to purchase stock in connection to the financing. The investment brings Neural Analytics’ total funding to approximately $66 million.Impact of Neurological DiseasesNeurological diseases such as stroke affects more than 795,000 people each year
Read More
RefleXion Medical Receives $60M Loan for Biology Guiding Radiotherapy Machine
RefleXion Medical, Inc., a biotargeting oncology company developing the first and only biology-guided radiotherapy (BgRT) machine* for targeted cancer treatment has received a $60M senior secured term loan from Oxford Finance LLC. The company plans to use the loan to support the completion of the FDA clearance process for its biology-guided BgRT system and its subsequent commercial launch.
Building from Positron
Emission Tomography
Positron emission tomography (PET) is an established
Read More