- Syqe Medical, Ltd. and TerrAscend Corp. announced an exclusive distribution agreement to bring Syqe’s flagship product, the SyqeTM Inhaler, to the Canadian market.
- TerrAscend will utilize their platform to both provide high-quality guidance to patients on use of the Syqe device and educate the medical community on the benefits of medical cannabis and the Syqe treatment.
- For the first time ever, the amount of THC in the bloodstream can be accurately controlled in a consistent,
Read More
Clinical Trials | News, Analysis, Insights - HIT Consultant
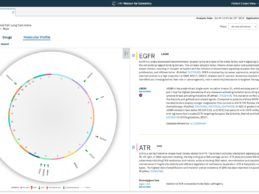
HUG Becomes First European Hospital to Adopt IBM Watson for Genomics
- Swiss healthcare provider Hôpitaux Universitaires Genève (HUG) becomes the first European university hospital to use IBM Watson for Genomics.
- IBM Watson for Genomics leverages AI to provide expertly validated, up-to-date, comprehensive content to enable clinical reporting & precision oncology programs.
- The HUG is Switzerland's leading center for influenza and emerging viral infections, as well as for childhood liver disease and pediatric liver transplantation.
Hôpitaux
Read More
Backed by Optum Ventures, Kaia Health Raises $8M for AI-Driven Digital Therapeutics for Back Pain & COPD
- Digital therapeutics startup Kaia Health raises $8M in funding led by Optum Ventures to support the accelerated execution of clinical trials required to demonstrate efficacy to the FDA.- Kaia Health utilizes multimodal therapy, which combines physical, psychological and educational elements to relieve back pain at home, without medication.- Optum’s investment in Kaia Health will consider use of Kaia Health’s digital therapeutic offerings for back pain and COPD with Optum and UnitedHealth
Read More
Ensuring A Healthy Approach to Big Data In The Life Sciences Industry
The old adage “knowledge is power” may seem cliched, but it definitely still rings true in the life sciences industry. In fact, maybe more so than in any other industry, healthcare insights are being driven by a vast array of critically important data from scientific research, clinical trials, and patient profiling. Teams are continually asked to identify, prioritize and develop promising therapies which are not only safe but innovative, effective and based on the latest research.
To achieve
Read More
TrialCard Acquires Digital Medication & Adherence Platform Mango Health
- TrialCard acquires digital medication management platform Mango Health to offer a best-in-class engagement and adherence solution to patients.
- The announcement marks the third acquisition for TrialCard in the past year.
- TrialCard Incorporated provides patient affordability, medication access and adherence, and patient support connecting over 30 million patients with nearly $12B in branded drug savings to date.
TrialCard
Incorporated announced it has entered an agreement to
Read More
UCI Institute for Clinical & Translational Awarded NIH Grant to Establish EHR-Integrated Clinical Data Warehouse
- National Institutes of Health (NIH) awards The Institute for Clinical & Translational Science at the University of California with a $25M grant over 5 years.
- Grant will be used to establish an EHR-integrated enterprise clinical data warehouse (CDW) to support healthcare breakthroughs.
The Institute for
Clinical & Translational Science at the University of California, Irvine
has been awarded $24 million over five years from the National Institutes of
Health (NIH) as part
Read More
Life Image Launches Real World Imaging for RWE Programs
- Life Image launches Real World Imaging (RWI) offering to respond to researcher needs for maturing insights and accelerating drug development decisions.
- Imaging data, especially for Real World Evidence (RWE), has historically been difficult, if not impossible, to access, retrieve, view, de-identify and normalize at-scale, even though it provides clinically material insights for a growing number of therapies.
- Life Image represents the only company providing large, heterogeneous,
Read More
Cedars-Sinai Named Coordinating Center for Stroke Preclinical Assessment Network
Patrick Lyden, MD, is leading a National Institutes of Health study about strokesQuick Glance- Cedars-Sinai named the coordinating center for the multicenter stroke research program- First-of-Its-Kind Program Will Help Doctors Analyze Several New Potential Stroke TreatmentsCedars-Sinai has been named the coordinating center for a multicenter stroke research program that will be the first of its kind in the U.S. Named the Stroke Preclinical Assessment Network (SPAN) will assess the effectiveness
Read More
UPenn Taps Medable’s Digital Trials Platform to Power LITE Psoriasis Study
The University of Pennsylvania School of Medicine has partnered with Medable, a provider of digital trials platform to accelerate patient enrollment and increase patient retention in the Light Treatment Effectiveness (LITE) study, which evaluates home versus office-based narrowband ultraviolet B phototherapy for the treatment of psoriasis.Phase 4 Pragmatic StudyMedable’s direct-to-patient app was selected for LITE, a Phase 4 pragmatic study that will enroll 1050 patients across 20-40 clinical
Read More
Report: Precision Medicine Is Impossible Without AI and Machine Learning
Artificial intelligence (AI) and machine learning are driving a great deal of the healthcare innovation in precision medicine, according to a new Chilmark Research report. The report reveals achieving the full potential of precision medicine is impossible to realize without applying AI and machine learning. Specifically, leveraging advanced machine learning and deep learning technology can rapidly analyze large datasets that outperform clinicians and researchers. Precision Medicine: Making
Read More