- Intermountain Healthcare launches new Kidney Care Center that will provide at-home dialysis with telehealth-enabled video visits to patients.
- Patients will be able to schedule kidney dialysis treatments that fit their schedule.
- The Intermountain Kidney Care Center will also focus on pre-emptive transplants, where patients are matched with living kidney transplant donors prior to end-stage renal failure.
Intermountain
Healthcare in Salt Lake City announced it is launching a
Read More
Kidney Disease
Healthy.io Nabs $60M for FDA-cleared Smartphone-Based Urinalysis for Chronic Kidney Disease
- Healthy.io raises $60M led by Corner Ventures for its FDA 510(k) cleared smartphone-based ACR test to be used in the aid of diagnosing chronic kidney disease (CKD).
- The funding round will be used to accelerate Healthy.io’s global expansion and product development.
- Healthy.io’s solution allows immediate electronic medical record (EMR) connectivity through the automated smartphone scan.
Healthy.io, the fast-growing Israeli digital health company that has turned
the
Read More
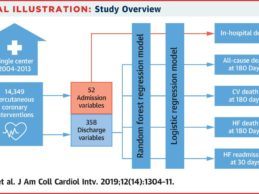
Machine Learning Algorithm Can Predict Which Cardiac Patients Are High-Risk Post Discharge
- New Mayo Clinic peer-reviewed study shows Medial EarlySign’s machine learning algorithm can predict which cardiac patients are at high-risk following discharge.
- The analysis was based on electronic health records (EHR), demographics, and social data collected from a cohort of 11,709 unique Mayo Clinic patients who underwent 14,349 PCIs during 14,024 hospital admissions.
- The study suggests AI solution can be more effective than traditional models to identify patients at risk of
Read More
How Will FY2020 IPPS Proposed Rule Affect Hospital CDI Programs?
In April, Centers for Medicare and Medicaid Services (CMS) released the fiscal year (FY) 2020 inpatient prospective payment systems (IPPS) proposed rule. The proposed rule notably includes approximately 1,500 complications or comorbidities (CC)/major complications or comorbidities (MCC) designation changes and 324 changes to International Classification of Diseases (ICD)-10-CM codes, along with several other updates. The designation changes are an effort to respond to the notion that the
Read More
How Tech Giants are Helping Providers Reinvent Patient Care
Healthcare costs are on the rise, and they show no signs of leveling off. According to the latest data available from the Centers for Medicare and Medicaid Services, healthcare spending in the United States reached $3.5 trillion in 2017, or $10,739 per person. Those costs are projected to rise by about five percent year-over-year.
As expenditures continue to skyrocket, healthcare providers are looking to new technologies and care strategies that can help them treat patients more effectively at a
Read More
NYC Health + Hospitals Launches Primary Care-Centered Diabetes Management Program
NYC Health + Hospitals, the largest public health care system in the nation announced the launch of a comprehensive, primary care-centered diabetes management program. The diabetes management program includes investing in new clinical pharmacy staff, equipment, and technology to improve health outcomes and expand services through telehealth techniques for more than 60,000 New Yorkers with diabetes who receive care in the City’s public hospitals and community-based health centers.Impact of
Read More
Kaiser Appoints First Chief Digital Officer, Teladoc’s New COO, Castlight Health CEO, Other Health IT Appointments
Kaiser Permanente has appointed Prat Vemana as its first chief digital officer for Kaiser Foundation Health Plan and Hospitals. In this newly created role, Vemana will lead the ongoing development and execution of Kaiser Permanente's digital vision and strategy, in collaboration with internal health plan, hospital, and medical group teams.
Atlantic Health System has named Dr. Sylvia Romm as Chief Innovation Officer. In her new role, Romm will be responsible for building new partner
Read More
AMA Grants New CPT Code for KidneyIntelX to Support Medicare & Reimbursement
The American Medical Association (AMA) has granted a CPT® Proprietary Laboratory Analyses (PLA) Code for Renayltix AI’s lead product, KidneyIntelX. The new code, 0105U, has been approved and published by the AMA CPT Editorial Panel, and is scheduled to become effective on October 1, 2019.Medicare Price for New CPT CodeA payment rate for the new code will be established for Medicare patients through the 2019 Clinical Lab Fee Schedule (CLFS) Annual Public Meeting process. Renayltix AI will shortly
Read More
Can Artifical Intelligence Solve The Chronic Kidney Disease Epidemic?
Chronic kidney disease (CKD) is a growing epidemic worldwide. Currently, there are 850 million individuals suffering from CKD globally, with 40 million in the United States alone. Of these individuals, 96 percent are not aware of having CKD, as kidney disease often exhibits no symptoms until it has progressed to a late stage.
As this epidemic continues to grow, healthcare providers and organizations are working to determine how artificial intelligence (AI) can be used to analyze electronic
Read More
EarlySign Unveils Commercial Availability of AI Diabetes Risk Predictors Algorithm
Medial EarlySign – an Israeli health AI company
has just announced the commercial availability of its first suite of machine
learning-based predictive diabetes risk solutions. Expanding the company’s
portfolio of clinical risk predictors, these new diabetes-focused AlgoMarkers
are designed to help healthcare systems identify and engage patients at high
risk for diabetes and downstream
Read More