What You Should Know:
Marks 8th FDA clearance for BlueStar, the only
reimbursable software as a medical device for diabetes that integrates with all
of a patient's existing devices.
Welldoc, revolutionizing digital health with the first FDA-cleared Software as a Medical Device (SaMD) for diabetes, announced today that the U.S. Food and Drug Administration (FDA) has cleared an additional feature for the digital health product BlueStar Rx which supports individuals using long-acting
Read More
FDA| Healthcare FDA Regulation | Policy, News, Analysis, Insights - HIT Consultant
Fitbit Unveils FDA-Approved Low-Cost Emergency Ventilator to Address COVID-19 Pandemic
What You Should Know:
- Today, Fitbit announced that it has received emergency use authorization (EUA) from the FDA for a new emergency ventilator named Fitbit Flow to help respond to the COVID-19 global health crisis.
- Fitbit plans to make the design available via open-source software to promote further collaboration and reach more people across the globe.
- Fitbit’s R&D, engineering and design teams worked quickly to apply their collective expertise in advanced sensor
Read More
Syapse Lands $30M to Accelerate Real-World Evidence in Oncology
What You Should Know:
- Syapse raises $30M in new equity funding to enable healthcare providers to deliver the best care to every cancer patient through precision medicine.
- Syapse partners with health systems, life sciences companies, and regulators to deliver clinical, programmatic, and research insights from the world’s largest network of health systems focused on precision medicine.
Syapse, a San
Francisco, CA-based provider of precision oncology
solutions announced $30
Read More
Propeller to Connect AstraZeneca’s Inhaler Symbicort Users to Propeller Platform
What You Should Know:
Propeller Health has been awarded FDA 510(k) clearance to
connect patients using AstraZeneca’s Symbicort Inhaler to Propeller, enhancing medication
coverage and respiratory digital health.
The sensor for Symbicort, a controller medication for
asthma and COPD, was developed in partnership with AstraZeneca. With this new
medication on our roster, Propeller now connects to 90 percent of the inhaled
medications for asthma and COPD on the U.S. market.
Propeller
Read More
Philips Awarded FDA Clearance for Wearable Biosensor to Monitor COVID-19 Patients
What You Should Know:
Wireless wearable biosensor (Philips Biosensor BX100) receives 510(k) clearance from the FDA and CE mark to help monitor COVID-19 patients in the hospital, with the first install at OLVG Hospital in the Netherlands
Philips Biosensor BX100 enhances Philips portfolio of devices, software, and services for identifying patients at risk for deterioration while limiting exposure to help improve staff and patient safety and preserve valuable personal protective equipment.
Read More
Why COVID-19 is Prompting Device OEMs to Partner with Software Developers
As COVID-19 continues to sicken thousands across the U.S., hospital staffs deserve every bit of praise they’re receiving for their service. Yet there’s another segment of the medical community on the front lines of the pandemic: medical device manufacturers.
While some forms of medical equipment have been marginalized or sidelined altogether, manufacturers of essential devices are working around the clock. According to an April 2020 Fortune Business Insights report, those with significant
Read More
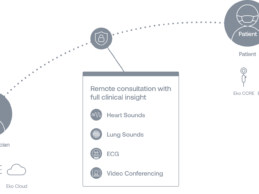
Eko Launches AI-Powered Telehealth Platform for Virtual Pulmonary and Cardiac Exam
What You Should Know:
Eko launches first AI-Powered telehealth platform for virtual
pulmonary and cardiac exams, providing clinicians with in-person level exam capabilities
during video visits.
Already deployed by more than 200 health systems for telehealth, platform goes beyond standard video conferencing to facilitate stethoscope audio, ECG live-streaming, and FDA-cleared identification of atrial fibrillation (AFib) and heart murmurs.
Eko today
announced Eko Telehealth, an
Read More
Collective Health Launches COVID-19 Workplace Solution to Facilitate Safer Workforce Reentry
What You Should Know:
- Collective Health launches COVID-19 workplace solution to help businesses reduce risk and facilitate safe work reentry
- As economic pressure continues to force broader re-openings across the U.S., Collective Go includes a testing ecosystem, occupational health protocol, mobile app, and HIPAA compliant platform.
Collective Health,
a San Francisco, CA-based employee healthcare company with an integrated
technology solution, today announced the launch of
Read More
Zebra Medical Vision Secures 5th FDA Clearance for Vertebral Compression Fractures AI Solution
What You Should Know:
- Israeli deep-learning medical imaging analytics startup Zebra-Med secures its fifth FDA clearance vertebral compression fractures AI solution for availability in the U.S.
- The AI solution will help health providers close the COVID-19-induced care gaps by identifying more patients at risk of osteoporosis, also known as the “silent killer,” costing over $52B in the U.S. alone.
- Zebra-Med is one of the first companies in the industry to provide an AI
Read More
FDA Approves Everlywell’s COVID-19 Test Home Collection Kit Using Nasal Swabs
What You Should Know:
- FDA approves Everlywell’s COVID-19 Test Home Collection Kit under the emergency use authorization (EUA) allows an individual to self-collect a nasal sample at home using Everlywell’s authorized kit.
- The Everlywell home-collection kit is currently the only authorized COVID-19 at-home sample collection kit for use with multiple authorized COVID-19 diagnostic tests.
The U.S. Food and Drug
Administration (FDA) has issued emergency use authorization (EUA) to
Read More