What You Should Know:
– Today, Fitbit announced that it has received emergency use authorization (EUA) from the FDA for a new emergency ventilator named Fitbit Flow to help respond to the COVID-19 global health crisis.
– Fitbit plans to make the design available via open-source software to promote further collaboration and reach more people across the globe.
– Fitbit’s R&D, engineering and design teams worked quickly to apply their collective expertise in advanced sensor technology, signal processing, robotics, hardware development and manufacturing — and consulted with clinicians at Oregon Health & Science University and MassGeneralBrigham Center for COVID Innovation, who are treating COVID patients — to create the device.
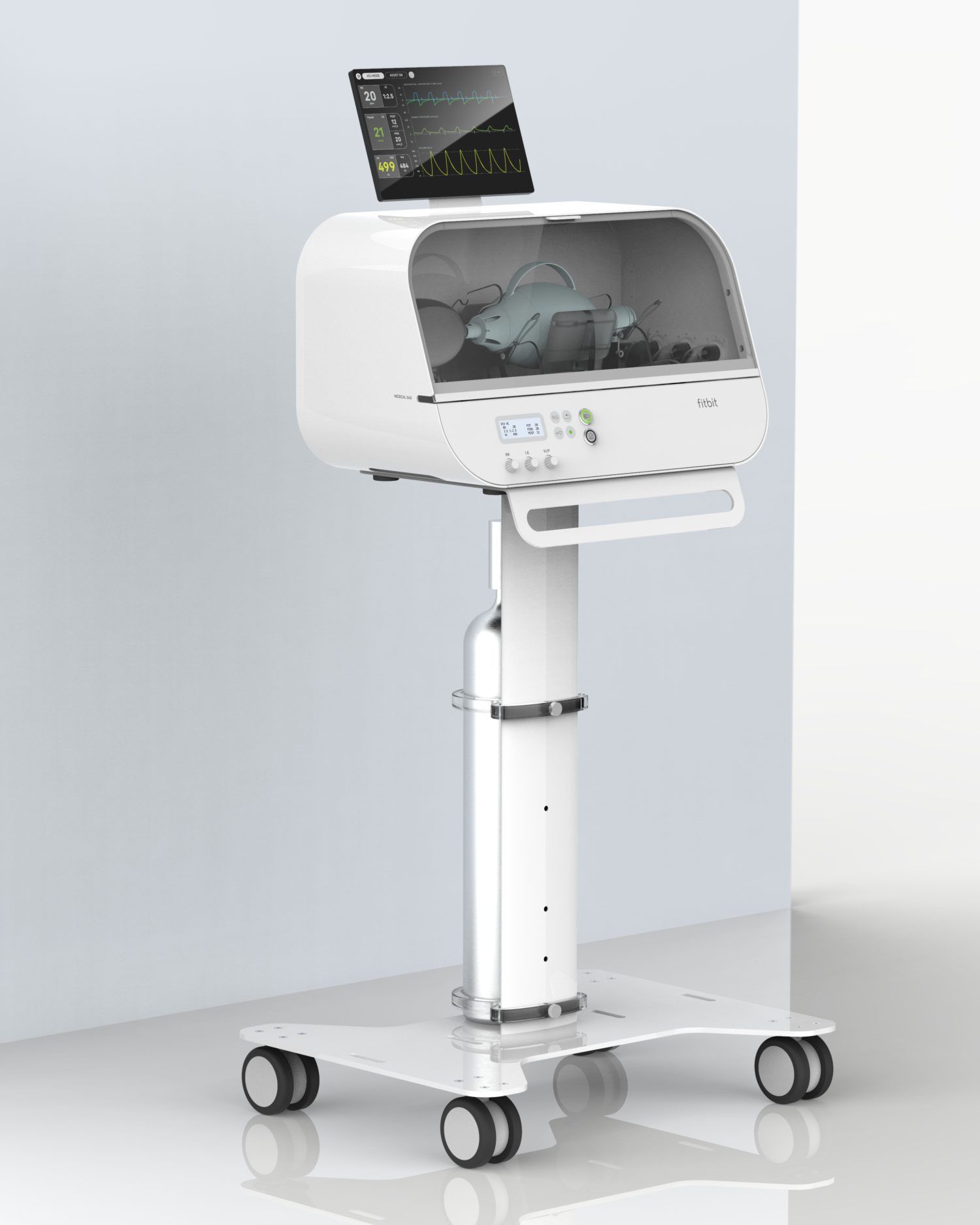
Fitbit, today unveils a new high-quality, low-cost, easy-to-use emergency ventilator, Fitbit Flow, which has obtained Emergency Use Authorization (EUA) from the U.S. Food & Drug Administration (FDA) for use during the COVID-19 public health emergency. The ventilator builds on standard resuscitator bags, like those used by paramedics, with sophisticated instruments, sensors, and alarms that work together to support automated compressions and patient monitoring.
Addressing the Global Need for Ventilators
According to the New England Journal of Medicine, “U.S. hospitals are already reporting shortages of key equipment needed to care for critically ill patients, including ventilators and personal protective equipment (PPE) for medical staff. Current estimates of the number of ventilators in the United States range from 60,000 to 160,000. No matter which estimate we use, there are not enough ventilators for patients with COVID-19 in the upcoming months.”
After seeing the global need for ventilators, Fitbit applied its deep in-house expertise in advanced sensor development and hardware design to quickly create Fitbit Flow, an automatic resuscitator inspired by the MIT E-Vent Design Toolbox and based on specifications for Rapidly Manufactured Ventilation Systems. During development and testing, Fitbit consulted with Oregon Health & Science University emergency medicine clinicians caring for COVID-19 patients at OHSU Hospital and worked with the Mass General Brigham Center for COVID Innovation working group on the design to meet the needs of practitioners.
“COVID-19 has challenged all of us to push the boundaries of innovation and creativity, and use everything at our disposal to more rapidly develop products that support patients and the health care systems caring for them,” said James Park, co-founder and CEO of Fitbit. “We saw an opportunity to rally our expertise in advanced sensor development, manufacturing, and our global supply chain to address the critical and ongoing need for ventilators and help make a difference in the global fight against this virus.”
Fitbit Flow Key Features
Fitbit Flow builds on standard resuscitator bags, like those used by paramedics, with sophisticated instruments, sensors, and alarms that work together to support automated compressions and patient monitoring. The device is designed to be intuitive and simple to use, potentially helping to reduce the strain on specialized staff who are typically needed to operate a commercial ventilator. Other similar emergency ventilators vary in the combination of features they offer, but Fitbit believes that none delivers all of the attributes of its device at the same lower price range.
“Fitbit Flow is a great example of the incredible innovation that emerges when academia and industry employ problem-based innovation to respond quickly to an important need. COVID-19 is a new illness and we still have much to learn about the progression, treatment, and potential recurrence of this disease. It’s critical that we develop solutions that can help ensure our health systems have the equipment they need now, and in the future if we do see a resurgence of COVID-19,” said David Sheridan,MD, MCR, Assistant Professor of Pediatric Emergency Medicine and Co-Director of Emergency Clinical Innovation Oregon Health & Science University.
Availability
Fitbit aims to leverage the its vast infrastructure and manufacturing capabilities that currently produces millions of Fitbit devices per year to produce large volumes of these emergency devices quickly. The goal is to supply these devices to healthcare systems around the world that do not have a sufficient number of traditional commercial ventilators. Fitbit Flow is designed to be used only when a traditional commercial ventilator is not available.
Fitbit is currently in talks with state and federal agencies to understand current domestic needs for emergency ventilators and plans to work with U.S. and global aid organizations as well, both today and ahead of any future waves of the virus.
