There’s an ongoing debate regarding the role that consumer health technology, like wearable health devices (i.e., smartwatches), can play in diagnostics, now and in the future. Because this is a relatively new technology, the scope of its potential impact is, at present, only scraping the surface.
Even so, smartwatches and their connected health apps are reshaping the healthcare industry. This technology has the ability to not only make personalized healthcare more widely accessible, but its
Read More
FDA| Healthcare FDA Regulation | Policy, News, Analysis, Insights - HIT Consultant
Ocutrx Acquires Spectrum AMT to Advance Capabilities for Medical Devices
What You Should Know:
Ocutrx Technologies, a global technology company developing medical devices with augmented reality (AR), extended reality (XR) and artificial intelligence (AI), acquires Spectrum Advanced Manufacturing Technologies, Inc., a Colorado-based electronics manufacturing and assembly company.With this acquisition, the consolidated Oxutrx_Spectrum company now has seven (7) process lines, including ISO Optics Lab capabilities,
Read More
FDA & Cerner Enviza Collaborate to Develop AI Tools for Drug Safety
What You Should Know:
- Cerner Enviza, an Oracle company, and FDA’s Sentinel Innovation Center are teaming up to develop AI tools to extract and assess clinical notes to better understand drug safety.
- Teaming with John Snow Labs, Cerner Enviza will use AI and NLP to develop tools to extract information from clinical notes within the EHR to better aid in better understanding the effects of medicines on large populations. The project known as the Multi-source Observational
Read More
Biostage Raises $6M to Advance Clinical Trials
What You Should Know:
Biostage, Inc., a cell-therapy biotechnology company with successful first-in-human experience in treating esophageal cancer and FDA approval to commence a clinical trial of the Biostage Esophageal Implant raises $6M from new and existing investors in a private placement of its shares of common stock.The funds will be used to accelerate the clinical development of Biostage’s lead product candidate, the Biostage Esophageal Implant, or BEI. The FDA has approved a
Read More
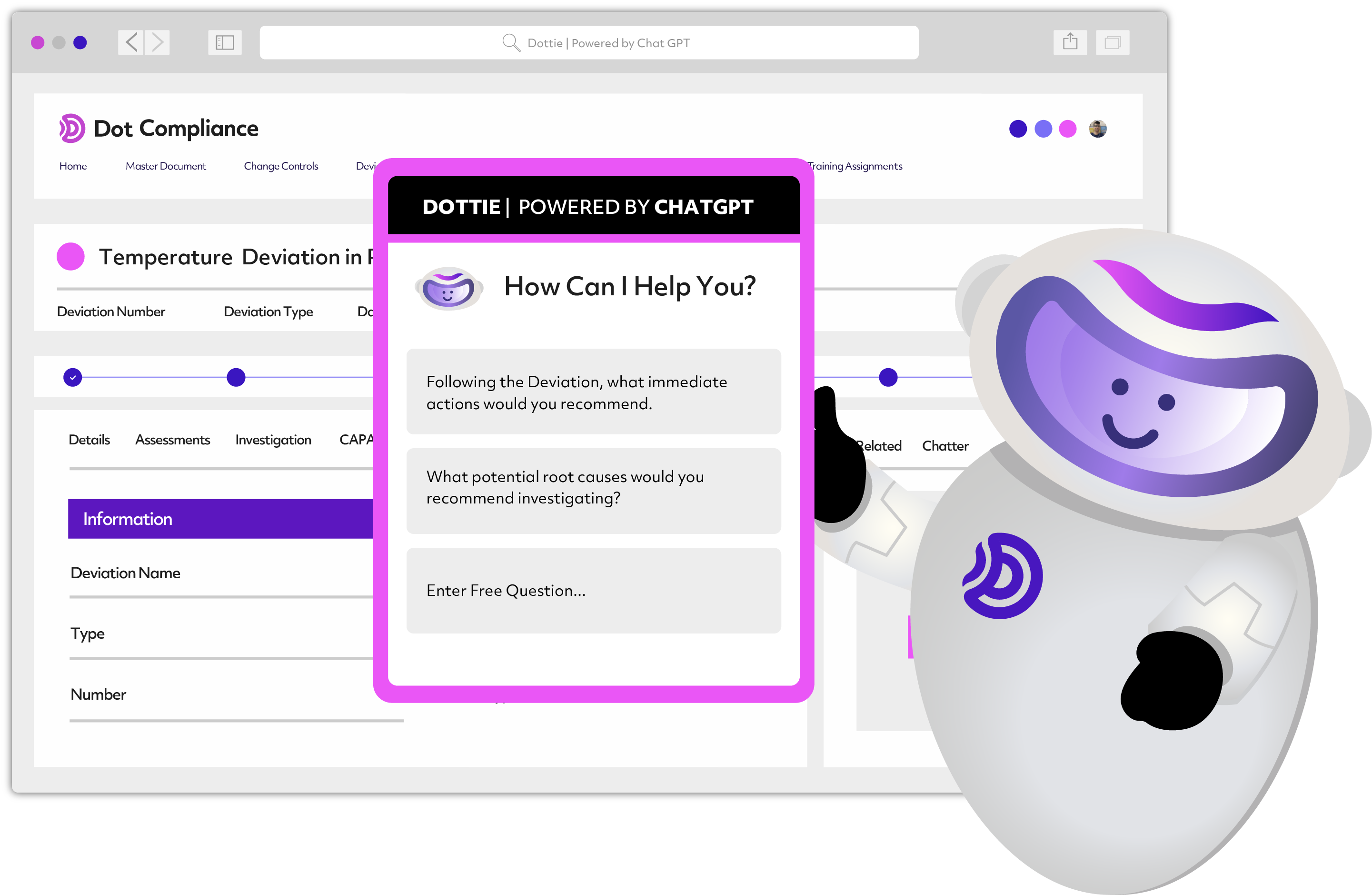
Dot Compliance Launches First AI Based ChatGPT Powered eQMS for Life Sciences
What You Should Know:
Dot Compliance, a leading provider of eQMS compliance solutions for the life sciences sector, has introduced an industry-first, ready-to-use AI-Based electronic Quality Management System for life sciences, powered by an embedded generative and predictive artificial intelligence.The system deploys ChatGPT combined with proprietary algorithms to optimize quality processes, automate tasks and help professionals working in quality assurance to focus on what really matters
Read More
OXOS Medical Raises $23M to Deliver ”Radiology Department in a Box”
What You Should Know:
OXOS Medical, the MedTech innovator developing simple and safe X-ray solutions, announced a $23 million Series A funding from Parkway Venture Capital and Intel Capital, bringing its total funding to $45 million.OXOS continues to build on its traction across outpatient clinics, the military, the Veterans Administration, sports teams, hospitals, imaging centers, and bioskills labs. To help accelerate the company’s growth, Gregg Hill, Parkway Venture Capital co-founder and
Read More
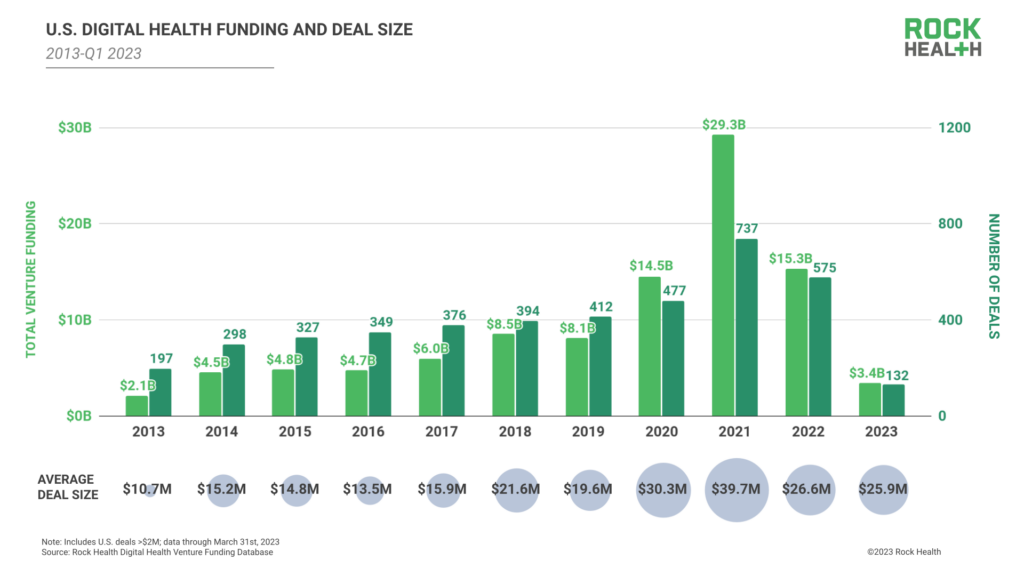
Q1 2023 Digital Health Funding Reaches $3.4B Across 132 Deals
What You Should Know:
2023 started off with the hallmarks of a rebound year. While Q4 2022 signaled the tail end of the digital health funding cycle, January and February funding numbers began to suggest that sector investment was slowly but surely inching back upwards. Inflation was easing ever so slightly. Investors were rediscovering their confidence and launching new projects, signaling optimism in the sector, according to a new Rock Health report. However, recent news—the collapse of
Read More
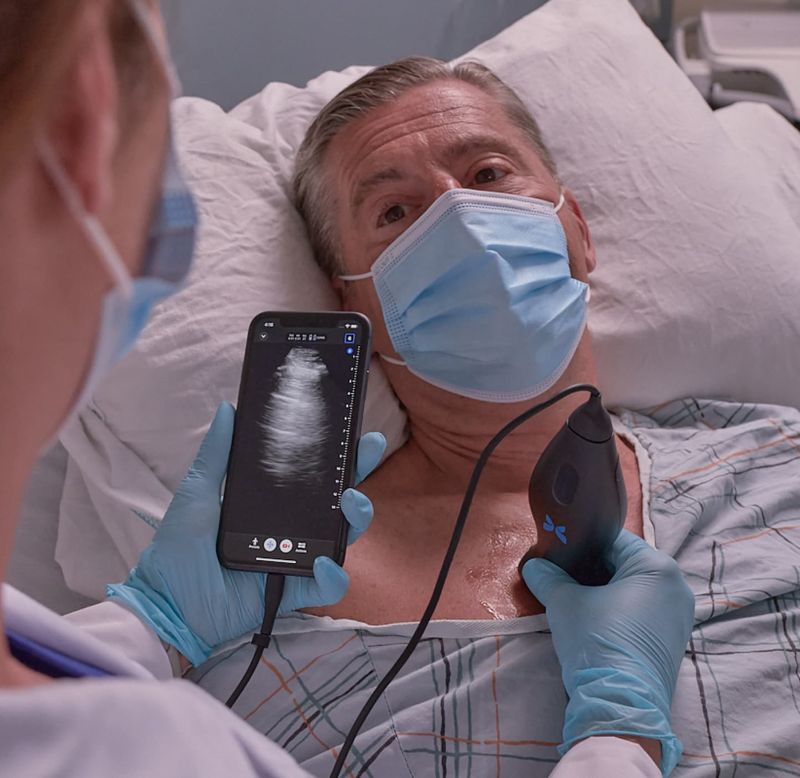
AI-Enabled Butterfly Network Lung Tool Receives FDA Clearance
What You Should Know:
- Today, Butterfly Network announced it received 510(k) clearance from the FDA for a groundbreaking AI-enabled tool named AI-enabled Auto B-line Counter that will help physicians assess adults’ lungs and accelerate providers’ abilities to make informed treatment decisions. Butterfly used data inputs from hundreds of sites across the country to train and develop its AI algorithms, offering potential for a broad and diverse range of age, gender, body mass index, ethnicity,
Read More
Inato Raises $20M to Make Clinical Trials More Inclusive
What You Should Know:
- Inato, a technology platform that connects pharma companies (trial sponsors) with community-based trial sites raises $20M in new funding (A2) led by Cathay Innovation with participation from existing investor Obvious Ventures and new investors La Maison and Top Harvest Capital. Inato works with many of the top 30 pharmaceutical companies worldwide to make clinical trials more inclusive.
- The new funding will fuel Inato’s continued product innovation, international
Read More
Exec Hires: Elucid Appoints Andrew Miller as CTO
What You Should Know:
- Elucid, Inc., a medical technology company providing physicians with AI-powered imaging analysis software to assess cardiovascular disease, has hired Andrew Miller as its chief technology officer (CTO).
- As CTO, Andrew will lead the technology strategy and oversee the engineering team’s execution. Elucid’s software is the only FDA-cleared non-invasive tool able to accurately characterize arterial plaque, simulating what pathologists would see under a microscope and
Read More