Augmented reality (AR) and virtual reality (VR) are redefining what's possible in healthcare, as well as other sectors. The 18 companies listed below are some of the top virtual and augmented reality companies in the medical industry.
Defining Augmented Reality
Augmented
reality is a technology that blends real-world elements with virtual ones. For
example, a person might visit a trade show and aim their smartphone at a
display to activate an AR experience that allows them to see
Read More
FDA Clearance
Welldoc Awarded 8th FDA Clearance for Bluestar Medical Device for Diabetes
What You Should Know:
Marks 8th FDA clearance for BlueStar, the only
reimbursable software as a medical device for diabetes that integrates with all
of a patient's existing devices.
Welldoc, revolutionizing digital health with the first FDA-cleared Software as a Medical Device (SaMD) for diabetes, announced today that the U.S. Food and Drug Administration (FDA) has cleared an additional feature for the digital health product BlueStar Rx which supports individuals using long-acting
Read More
Philips Awarded FDA Clearance for Wearable Biosensor to Monitor COVID-19 Patients
What You Should Know:
Wireless wearable biosensor (Philips Biosensor BX100) receives 510(k) clearance from the FDA and CE mark to help monitor COVID-19 patients in the hospital, with the first install at OLVG Hospital in the Netherlands
Philips Biosensor BX100 enhances Philips portfolio of devices, software, and services for identifying patients at risk for deterioration while limiting exposure to help improve staff and patient safety and preserve valuable personal protective equipment.
Read More
Zebra Medical Vision Secures 5th FDA Clearance for Vertebral Compression Fractures AI Solution
What You Should Know:
- Israeli deep-learning medical imaging analytics startup Zebra-Med secures its fifth FDA clearance vertebral compression fractures AI solution for availability in the U.S.
- The AI solution will help health providers close the COVID-19-induced care gaps by identifying more patients at risk of osteoporosis, also known as the “silent killer,” costing over $52B in the U.S. alone.
- Zebra-Med is one of the first companies in the industry to provide an AI
Read More
FDA Expedites Clearance for AI Ultrasound Solution to Fight COVID-19
What You Should Know:
- FDA expedites clearance of Caption Health’s AI-powered ultrasound solution to aid frontline healthcare workers in the fight against COVID-19 pandemic.
- Caption Health’s AI-guided imaging supports the assessment of cardiac function and decreases personnel exposure to novel coronavirus.
In response to the growing body of evidence demonstrating
the impact of COVID-19
on the heart, the U.S. Food and Drug Administration (FDA) has expedited
clearance of an update
Read More
Lumos Diagnostics Raises $15M to Expand Point of Care Diagnostic Solutions Internationally
- Lumos Diagnostics raises $15M in Series A funding to support the international expansion of its point of care diagnostic solutions.
- Lumos provides assay development and manufacturing services for customized POC tests as well as directly develops, manufactures and will commercialize a suite of proprietary Lumos-branded POC tests that focus on the systemic host immune response.
Lumos Diagnostics, a Sarasota, FL-based health IT company, today announced it has raised $15M in Series A
Read More
Sony Awarded FDA Clearance for Smart Operating Room Platform in the U.S.
- Sony announces FDA 510(k) clearance from the FDA for NUCLeUS™, the smart operating room platform, for sale in the U.S.
- NUCLeUS provides an enhanced, streamlined workflow for operating rooms and clinical spaces with direct access to imaging data from an easy-to-use central dashboard.
Sony Electronics has received FDA 510(k) clearance from the
Food and Drug Administration for the company’s NUCLeUS™
Operating Room, Imaging Management and Collaboration Control platform.
Proven in
Read More
BioIntelliSense Awarded FDA 510(k) Clearance for On-Body Sensor for Scalable Remote Care
- BioIntelliSense announces FDA clearance of the BioSticker, the first single-use medical device enabling 30 days of continuous vital signs monitoring. - The FDA-cleared BioSticker medical device is designed to be discreetly worn on the upper left chest for effortless remote data capture and a simplistic “stick it on and forget it” patient experience. BioIntelliSense, Inc., a continuous health monitoring and clinical intelligence company, today announces the U.S. commercial launch of its
Read More
FDA Breakthrough Status Granted for Heart Failure Algorithm by Eko
- Eko announced the FDA has granted “breakthrough status” for an algorithm that could provide an easily accessible screening test for heart failure during routine physical exams.- This designation is awarded to novel innovations that demonstrate the potential to address unmet medical needs for life-threatening or irreversibly debilitating diseases. - Heart failure is often left untreated until it’s too late due to costs, inaccessibility to echocardiogram testing, or misdiagnosis. With nearly six
Read More
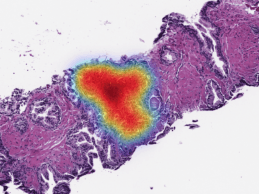
Paige Raises $45M to Expand AI-Native Digital Pathology Ecosystem to Accelerate Biomarker Discovery
- Paige raises $45M in Series B funding led by Healthcare Venture Partners with participation from Breyer Capital, Kenan Turnacioglu, and others. - The funding will be used to accelerate commercial efforts of its AI-native digital pathology ecosystem in the U.S., Europe, Brazil, and Canada. Paige, a NYC-based leader in computational pathology transforming the diagnosis and treatment of cancer, today announced it has closed its Series B funding round of $45 million, bringing the Company’s
Read More