What You Should Know:
- Thirona, a pioneer in AI-powered lung analysis, received a major boost for its cutting-edge technology LungQ™ with the U.S. Food and Drug Administration (FDA) 510(k) clearance for its latest update, v3.0.0.
- This FDA clearance paves the way for American hospitals to now harness the software's advanced capabilities, marking a significant step forward in lung disease diagnosis and treatment.
LungQ 3.0.0: Precision Navigation for Lung Interventions
This latest
Read More
FDA clearance 510k
FemTech: Mosie Baby Awarded FDA Clearance for At-Home Intravaginal Insemination
What You Should Know:
Mosie Baby, a pioneering at-home fertility care company, has secured FDA Class II clearance for its Mosie Baby Kit making it the first and only FDA-cleared over-the-counter kit for use in intravaginal insemination (IVI).The kit was created to support those who are unable to conceive with intercourse or for whom intercourse is not an option. Following its FDA 510k Class II clearance, Mosie Baby looks forward to expanding access to its Mosie Baby Kit which was
Read More
RapidAI Receives FDA Clearance for Rapid SDH (Subdural Hematoma) for Trauma Care
What You Should Know:
- RapidAI has received FDA clearance for Rapid SDH, its AI-powered module for the detection and notification of suspected hemispheric acute and chronic subdural hematoma.
- The need for the RapidAI solution is urgent, with SDH cases in US patients expected to increase by nearly 80% before 2040.
AI Module for Detection of Hemispheric Subdural Hematomas
Rapid SDH leverages AI to help neurocritical care teams identify suspected hemispheric subdural hemorrhage
Read More
Welldoc Awarded FDA 510(k) Clearance for Diabetes Platform
What You Should Know:
Welldoc®, a digital health leader revolutionizing chronic care, today announced the receipt of its 11th 510(k) clearance from the Food and Drug Administration (FDA) for its award-winning diabetes digital health solution, BlueStar®.This clearance immediately follows Welldoc’s 10th 510K clearance announcement earlier this month, solidifying the company’s continued leadership in chronic care innovation.
A Continuous Glucose Monitoring (CGM) Informed Bolus Insulin Dose
Read More
Viz.ai Awarded 510(k) Clearance for AI-Powered Cerebral Aneurysm Detection Solution
What You Should Know:
- Viz.ai, a San Francisco-based provider of AI-powered intelligent care coordination for stroke, has received FDA 510(k) clearance for Viz ANEURYSM (formerly Viz ANX), the first and only AI-powered cerebral aneurysm detection solution designed to facilitate population screening and enhanced care management.
- The new algorithm uses AI to detect suspected cerebral aneurysms, enabling hospital systems
Read More
Biotricity Awarded FDA 510(k) Clearance for Cardiac Monitoring Device
What You Should Know:
- Biotricity, Inc. (NASDAQ:BTCY), a medical diagnostic and consumer healthcare technology company, today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Biotres Cardiac Monitoring Device, a three-lead device for ECG and arrhythmia monitoring that is intended for lower-risk patients.
- Most remote cardiac monitors are passive — they don’t record ECG and other cardiac data in real-time — and lack cross-compatibility
Read More
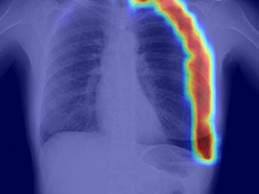
RADLogics Secures FDA Clearance for AI-Powered Chest X-Ray App for Triage & Prioritization
What You Should Know:
- RADLogics today announced that it has secured
510(k) clearance from the FDA for the company’s novel AI-Powered chest X-ray pneumothorax application,
which identifies and prioritizes chest X-ray scans that appear
to contain a pneumothorax, a collapsed lung, for urgent radiologist review.
- RADLogics’ FDA cleared CT and X-ray solutions
– including this application – are
Read More
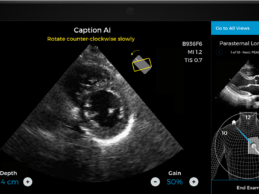
Caption Health AI Awarded FDA Clearance for Point-of-Care Ejection Fraction Evaluation
What You Should Know:
- Caption Health AI is awarded FDA 510(k) clearance for
its innovative point-of-care ejection fraction evaluation.
- Latest AI ultrasound tool makes it even easier to
automatically assess ejection fraction, a key indicator of cardiac function, at
the bedside--including on the front lines of the COVID-19 pandemic.
Caption Health, a Brisbane,
CA-based leader in medical AI technology, today announced it has received FDA
510(k) clearance for an updated version of
Read More
Zebra Medical Vision Secures 5th FDA Clearance for Vertebral Compression Fractures AI Solution
What You Should Know:
- Israeli deep-learning medical imaging analytics startup Zebra-Med secures its fifth FDA clearance vertebral compression fractures AI solution for availability in the U.S.
- The AI solution will help health providers close the COVID-19-induced care gaps by identifying more patients at risk of osteoporosis, also known as the “silent killer,” costing over $52B in the U.S. alone.
- Zebra-Med is one of the first companies in the industry to provide an AI
Read More
FDA Clears AI-Powered EchoGo Core for Early Detection of Cardiovascular Disease
- Ultromics has just become one of the first companies to have 510(k) FDA clearance for an AI medical device. - EchoGo will now be available for clinicians in the UK and US to use to help them identify cardiovascular disease earlier. - EchoGo automates cardiac measurements and is the first AI application to measure cardiac strain, improving patient care and outcomes.Ultromics, the UK-based health technology firm at the forefront of applying artificial intelligence to echocardiography has
Read More