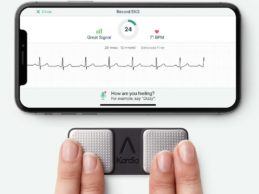
What You Should Know:
- AliveCor announced they received FDA clearance of new
algorithms for use with their personal EKG devices, KardiaMobile and
KardiaMobile 6L. These additional determinations will be available via a
software upgrade for the Kardia devices in 2021.
- The additional FDA-cleared algorithms double the number
of heart rhythm disturbances that AliveCor’s Kardia devices can detect,
broadening the number of patients who are able to use their remote
Read More
EKG Medical Devices
Security Vulnerabilities Detected in ICDs: Addressing Ransomware Risk in Medical Devices
Medical device usage is on the rise, and that reality potentially puts people at risk if those gadgets have security flaws. It's crucial for device manufacturers, industry regulators and health practitioners to work together to ensure safety are paramount as adoption rises.Internal health devices are particularly at risk, especially since patients and providers may not immediately notice issues. The people who use or prescribe them assume they'll work as expected and typically don't have ways to
Read More
AliveCor Receives “Breakthrough Device” Designation from FDA for Bloodless Hyperkalemia Test
AliveCor, a Mountain View, CA-based digital health startup developing solutions to transform cardiac care, announced on Monday received “breakthrough device” designation from the U.S. Food and Drug Administration for their bloodless hyperkalemia test. Developed with Mayo Clinic, AliveCor’s KardiaK Platform screens for elevated levels of blood potassium – hyperkalemia, without requiring any blood from the patient.This designation signals that the FDA will consider the technology on an
Read More
FDA Clears AliveCor’s KardiaBand for Apple Watch, Records Medical-Grade EKG in 30 Seconds
AliveCor, a FDA-cleared personal electrocardiogram (EKG) technology, today announced FDA clearance of KardiaBand in the U.S., allowing Apple Watch users to discreetly capture their EKG anytime, anywhere in order to quickly detect normal sinus heart rhythms and atrial fibrillation (AFib), the most common heart arrhythmia. The announcement marks first FDA-cleared medical device accessory for the Apple Watch, KardiaBand can record an EKG in 30 seconds with just a touch of its integrated sensor.
Read More
AliveCor Unveils Medical-Grade EKG Band for Apple Watch
AliveCor, Inc., a provider of FDA-cleared electrocardiogram (EKG) technology for mobile devices has unveiled the first medical-grade EKG band for the Apple Watch, Kardia™ Band. Pending 510k clearance, the Kardia™ Band allows people to discretely capture their EKG anytime, anywhere. The band is expected to ship late spring with a new app now available for smartphones.
How It Works
Users can record a single-lead EKG by simply touching Kardia Band's integrated sensor that communicates with
Read More