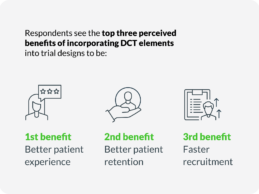
What You Should Know:
- New data from Tufts and Medable reveal on average, decentralized clinical trials (DCTs) are associated with reduced clinical trial timelines and can achieve net financial benefits ranging from five to 14 times for Phase II and Phase III trials, respectively.
- The findings are based on financial modeling and analysis of trial data from the Tufts Center for the Study of Drug Development (CSDD), Tufts University School of Medicine, and more than 150 clinical trials
Read More
Clinical Trials | News, Analysis, Insights - HIT Consultant
GSK Launches Accelerated Clinical Trial with Tempus
What You Should Know:
- Tempus and GlaxoSmithKline (GSK) announced an accelerated phase II study evaluating the efficacy and safety of niraparib, an oral, once daily PARP inhibitor, for patients with breast and pancreatic cancers. The study, titled “Niraparib in the Treatment of Patients With Advanced PALB2 Mutated Tumors” (the PAVO study, NCT05169437) is sponsored by Tempus and opened for enrollment on January 7, 2022.
- This trial’s data-driven design and site selection
Read More
Northwell Launches Virtual Clinical Trial to Reduce Cardiovascular Disease in Black Community
What You Should Know:
- The Feinstein Institutes for Medical Research, the science arm of Northwell Health, recently received a $150k grant from TD Bank, America's Most Convenient Bank, to launch a new research program and the first clinical trial to reduce cardiovascular disease in the Black community and determine the best behavioral interventions to lower Cardiovascular disease (CVD).
Impact of Cardiovascular Disease in Black Community
Cardiovascular disease (CVD) is a major driver
Read More
70% of US Oncologists Are Wrong About Clinical Trial Diversity
What You Should Know:
- COTA's study reveals that 70 percent of oncologists believe patient populations enrolled in clinical trials for cancer research are representatively diverse.
COTA, Inc., an oncology real-world data and analytics company, announced insights from a study of practicing oncologists that reveals 83 percent of oncologists surveyed believe real-world data is critical to accelerating the development of potentially life-saving cancer drugs and treatments. The study, which
Read More
77% of Sponsors, CROs Plan to Run Agile Clinical Trials in Next Months
What You Should Know:
- Today Science 37 published a new report suggesting a momentous shift in the way that clinical trials are conducted.
- For the first time, more research sponsors and CROs are planning to run Agile/Hybrid Clinical Trials than ever before. Findings show that the vast majority or 77% of respondents plan to run an agile clinical trial in the next 12 months, compared to 59% for the previous 12 months.
Science 37 Holdings, Inc. (Nasdaq: SNCE) (“Science 37”), a
Read More
Overcoming Key Hurdles in Decentralized Trials with Better Education
As with countless global industries, clinical trials were forced to move key functions online as COVID-19 swept the world in early 2020. Clinical research operations had to rapidly deploy remote approaches that were previously in the planning or pilot stages. While the uptake was rushed, the results have been largely positive. Decentralized clinical trials, and their near cousins that apply a hybrid approach of in-person and remote visits, are increasingly seen as a viable solution for biopharma
Read More
Decentralized Clinical Research Will Be A Paradigm-Shifting Trend
The field of clinical research is on the precipice of change, and for good reason. Traditional clinical trials and processes are primarily confined to sterile, isolated clinical settings that are often narrow in scope and provider-centric. Researchers of tomorrow, however, already are identifying accurate and effective new methods to conduct and draw meaning from their work, after concluding that current models for clinical research are not capturing participants’ real-world, lived experiences
Read More
3 Barriers to Decentralized Clinical Trials Adoption
It’s official. Decentralized clinical trials have disrupted the clinical trial process for good. The COVID-19 pandemic elevated many digital innovations, proving that virtually connecting with patients is not only convenient but necessary when it comes to providing equitable access to cutting-edge treatments and clinical research.
Historically, traditional clinical trials have always experienced recruitment and retention challenges due to inconveniently located trial sites, lack of awareness
Read More
Decentralized Clinical Trials: Keys to Optimizing Diversity and Inclusion
The U.S. pharma industry and research intuitions have long struggled with increasing clinical trial diversity in an effective, sustainable, and scalable fashion. Clinical research, in general, acknowledges the universal struggle of recruiting enough participants from various demographic groups. For example, racial and ethnic minorities have been historically underrepresented in clinical trials—a problem that still persists today.
According to Deloitte, African Americans comprise 13% of the
Read More
Medable Secures $304M at $2.1B Valuation for Decentralized Clinical Trials Platform
What You Should Know:
- Decentralized Clinical Trials (DCT) leader Medable, today announced a $304M Series D funding round today – the company’s largest and fourth funding round in 18 months, bringing total capital raised to $521M – and valuation to $2B+. The funding round was co-led by new investors Blackstone Growth and Tiger Global and existing investor GSR Ventures. The round also includes follow-on investment from existing investors Sapphire
Read More