I have dedicated my career to clinical research and making strides to advance modern medicine. In the work I do as a clinical researcher, we are addressing racial and gender biases that exist today in clinical trials. For example, in cardiovascular disease—my area of study—we know that women’s symptoms present differently than men and yet their symptoms have often gone undiagnosed or dismissed by physicians.
It is well documented that communities of color have historically been mistreated by
Read More
Life Sciences | News, Analysis, Insights - HIT Consultant
Lightship Raises $40M to Bring Decentralized Clinical Trials at Scale
What You Should Know:
- Lightship, a virtual-first provider of clinical trials, today announced that it has raised $40M to create access to clinical research for all patients and improving diversity among patient groups taking part in clinical trials around the world led by Define Ventures and Brook Byers, with participation from Khosla Ventures, McKesson Ventures and Marc Benioff’s TIME Ventures. This round of investment follows $20M of funding that Lightship announced in February 2020 to
Read More
3 Ways Digital Health is Transforming Decentralized Clinical Trials
Decentralized Clinical Trials (DCTs) have been gaining steam over the last decade, but it was the COVID-19 pandemic that cast its advantages into the limelight. Sponsors benefit by creating efficiencies virtually throughout drug discovery, research, and treatment phases. However, the greatest long-term benefactors will ultimately be the patients.
Many aspects of traditional clinical trials are inconvenient for potential candidates, including the commute distance to trial sites, time
Read More
RWE: Supporting Regulatory Submissions with Real-World Evidence
In 2016, the 21st Century Cures Act was signed into law, mandating that the FDA “establish a program to evaluate the use of real-world evidence (RWE) to help support the approval of new indications for a drug and to help to support or satisfy post-approval study requirements.” Along with advances in the availability and quality of real-world data (RWD) from sources like electronic health records (EHRs), registries, medical claims and pharmacy data, the Cures Act has been a catalyst for increased
Read More
Medidata, Labcorp Partner to Expand Decentralized Clinical Trials for Digital Biomarker Discovery
What You Should Know:
- Medidata, a Dassault Systèmes company, today announced a first-of-its-kind partnership with Labcorp using the Medidata Sensor Cloud to introduce a new model to advance the use of medical-grade sensors in clinical trials and accelerate biomarker discovery.
- As part of the partnership, Medidata will receive and process medical-grade sensor data within drug, vaccine and device trials across Labcorp Drug Development’s clinical trial portfolio,
Read More
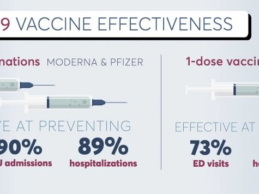
EHR Data Reveals COVID-19 Vaccines Effective in Preventing Hospitalizations, ER Visits
What You Should Know:
- Real world evidence from electronic medical records (EHR) data reveals the COVID-19 vaccines are highly effective at preventing hospitalizations, emergency department visits and intensive care admissions due to the virus, according to a study just published in the New England Journal of Medicine (NEJM).
- The study, “Effectiveness of COVID-19 Vaccines in Preventing Ambulatory and Inpatient Care” analyzes actual data from hospitals around the US and shows
Read More
Cedars-Sinai Cancer Launches ‘Molecular Twin’ Initiative for Precision Cancer Treatment
What You Should Know:
- Cedars-Sinai Cancer and Tempus, a provider of artificial intelligence and precision medicine, are harnessing the power of big data and AI to design personalized cancer treatment options by creating virtual replicas of patients' DNA, RNA, protein and other information to help identify the most effective approach to each individual's disease.
- By creating these "molecular twins," scientists can genetically classify cancer genes and proteins of particular tumors
Read More
H1 Launches Solution to Build More Inclusive, Successful Clinical Trials
What You Should Know:
- H1, which provides the largest global healthcare platform connecting the healthcare ecosystem, announced today that it has launched H1 Trial Landscape, the first solution that not only helps pharma and life sciences companies determine the required clinical experience levels for their clinical trials, but also enables them to find the best patient segments to improve inclusivity, retention and recruitment for successful trials.
- Studies have shown that more than
Read More
Clinical Trials: 4 Strategies to Maximize Patients Referrals
Clinical trials were front and center in 2020, as pharma companies raced to develop and prove the safety and efficacy of their COVID-19 vaccines. Despite this increased awareness, many potential participants and referring clinicians remain unaware of available trials and how to become involved. This negatively impacts enrollment efficiency and overall study timelines.
Enter patient referral platforms. The growth of these third-party services has helped bridge the knowledge gap for both
Read More
Fresenius Medical Invests Additional $25M in Biotech Platform Humacyte
What You Should Know:
Fresenius Medical Care (FMC) today announced its plans to invest an additional $25 million in clinical-stage biotechnology platform company Humacyte following the extension of its collaboration in June. Fresenius Medical Care acquired a stake in Humacyte in 2018 for $150M as part of a strategic partnership.
Following the merger of Humacyte with a special purpose acquisition company (SPAC), FMC is increasing its position in the newly combined
Read More