What You Should Know:
Substance use disorder has far-reaching impacts across society, and healthcare workers are not immune. In some cases, healthcare worker addiction can lead them to use prescription drugs intended for patients or steal them to be sold for personal benefit.A recent survey sponsored by Invistics, acquired by Wolters Kluwer Health earlier this year, found that despite 98% of healthcare executives agreeing that drug diversion occurs in hospitals, nearly four in
Read More
Life Sciences | News, Analysis, Insights - HIT Consultant
Integral Secures $6.9M to Expand Real-Time Compliance for Life Sciences
What You Should Know:
- Integral, an innovator in safeguarding privacy and maximizing data quality raises $6.9M in seed funding to protect sensitive health data and provide automated expert certification software that enables health and life sciences companies access to compliant, rich, de-identified data in hours, rather than months.
- The seed round was led by Haystack, The General Partnership, and Virtue Ventures, with additional support from Also Capital, Array Ventures,
Read More
The Self-Driving Clinical Trial: Optimizing Research with AI and ML
The power and potential of artificial intelligence (AI) and machine learning (ML) in the healthcare industry are unparalleled. The use of automated technology systems like digital workflows, orchestration and storing of data digitally is helping to shape their application, enabling far greater personalization of treatment for patients and greater efficiency and speed of clinical trials.
As the amount of health data increases, the possibility of establishing a self-driving clinical trial is
Read More
QuantHealth Secures $15M for AI-Powered Clinical Trial Design
What You Should Know:
- QuantHealth, an Israeli-based AI-powered clinical trial design startup raises $15M in a Series A funding round co-led by Bertelsmann Investments and Pitango HealthTech, with participation from existing investors Shoni Top Ventures and Nina Capital.
- The round also includes participation from previous investors including Boston Millennia Partners, Atooro Fund, and Renegade Ventures. The latest round of funding brings QuantHealth to $20M in funding to
Read More
AmerisourceBergen Rebrands as Cencora
What You Should Know:
AmerisourceBergen Corporation (NYSE: ABC), announced the rebranding of its name and stock ticker change to Cencora, Inc. (NYSE: COR).Under the new name Cencora, the company unifies its 46,000 employees – across several global business segments – under one identity, propelled by a bold vision and purpose-driven approach to creating healthier futures.Cencora will continue to invest in and focus on its core pharmaceutical distribution business, while also growing its
Read More
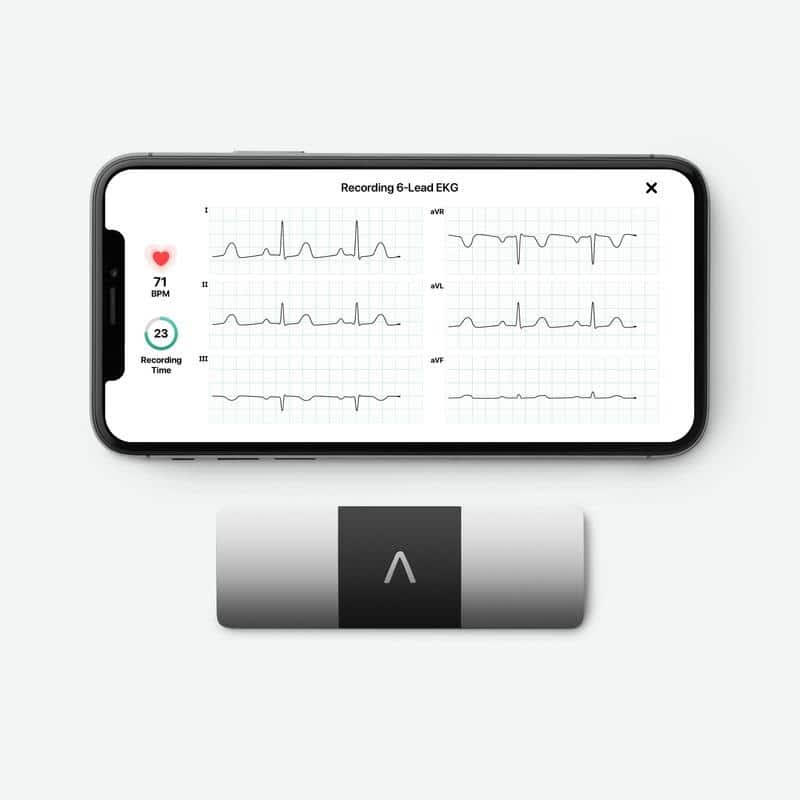
AliveCor and Clario Partner to Offer Decentralized Clinical Trials- Focused Six-Lead ECG Solutions
What You Should Know:
Clario announced an expansion to its collaboration with AliveCor to enable trial participants to collect medical-grade six-lead ECG readings in the comfort of their own homes through KardiaRx, a clinical-trial-focused app. This technology enables pharmaceutical and biotech customers to consider decentralized and hybrid clinical trial strategies in study protocols that would have otherwise been limited to on-site evaluations.
Enabling Accurate Cardiac Safety
Read More
Nebraska Medicine and Helix Launch Genomic Testing Statewide
What You Should Know:
Helix, the leading population genomics and viral surveillance company in the nation, and Nebraska Medicine, the state’s largest hospital and leading academic health network, announced a partnership today to launch a population genomics program to drive precision medicine for all individuals in Nebraska called the Genetic Insights Project.The research program will identify participants’ risk for a variety of cancers and other potentially life-threatening
Read More
Transforming Healthcare & Life Sciences with 5G Connectivity
With its rapid adoption, 5G technology has the potential to completely revolutionize customer experiences and boost revenue in the life science industry.
Accenture research found that companies which invest in 5G capabilities will grow revenue 2.5 times faster in the next three years. And the life sciences industry is taking notice – according to a survey by HFS Science, the healthcare industry is positioned as the second-largest market for adopting 5G technology. PwC predicts that
Read More
Drug-Gene Interactions: Minimizing Risk with Pharmacogenomics
Every individual responds differently to medications, and their reactions can vary greatly. While a specific drug may be effective for one person with no adverse effects, another individual may experience a negative reaction to the same medication. Pharmacogenomic (PGx) testing enables the identification of patients who are prone to adverse drug reactions, individuals who are more likely to benefit from specific medications and those who may require adjusted dosages. As adverse drug reactions
Read More
Q/A: AiCure SVP Talks How Digital Health Can Improve Access to Clinical Trials
The imperative to enhance access to quality care and research is a central concern gripping both the healthcare and pharmaceutical sectors. At the heart of this challenge lies the crucial necessity for clean and streamlined patient data, which currently suffers from inadequacies in comprehensiveness and accuracy. Addressing this pivotal issue carries immense benefits, particularly in the sphere of targeted trial recruitment efforts. By harnessing better patient data records, the possibilities
Read More