What You Should Know:
- SyTrue offers its free AI-based solutions free of charge to qualified global public health organizations to help researchers and governments battling the COVID-19 pandemic
- SyTrue has developed solutions that enable identification of disease trends, early detection, and interventions, safety-monitoring of medicinal therapies, COVID-19 knowledge extraction, and accelerated identification of patient cohorts.
The solution will support efforts to identify
Read More
Public Health
6 Benefits of Telemedicine in Managing the Diabetes Epidemic
The American Diabetes Association reveals that 9.4% of the American population has diabetes, and with numbers that high nearly all Americans have a friend or family member with the disease. However, many Americans are not aware that telehealth can enable diabetic patients to manage their condition—treatment that would have otherwise been difficult to come by for rural patients with limited access to care.
With such a high prevalence among the population, providing care regardless of proximity
Read More
Travel Nurse Pay Nearly Doubles Nationwide from Coronavirus Outbreak
What You Should Know:
- As demand for nurses across the country increases to treat the coronavirus outbreak, the average weekly pay for travel nurses has nearly doubled, according to recent findings from healthcare staffing platform NurseFly.
- There was a 76% increase in average nurse pay nationwide since March, with increases of up to 90% over the average pay in Washington.
- Hospitals are paying Crisis/Pandemic rates up to $4,400 weekly to quickly staff up for the caring of
Read More
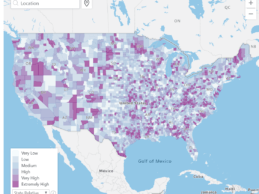
Wolters Kluwer Launches Global COVID-19 Search Intensity Monitoring Map
What You Should Know:
- Wolters Kluwer arms coronavirus efforts with the launch of a global COVID-19 Search Intensity Monitoring Map using UpToDate that shows how often COVID-19 topics are searched by doctors, nurses and other clinicians globally.
- Cross-disciplinary teams at Wolters Kluwer developed the interactive map by analyzing how often specific questions related to COVID-19 are searched by doctors, nurses and other clinicians globally. This leverages global search activity
Read More
Charges for All Hospitalized COVID-19 Patients May Reach Up to $1.4 Trillion
What You
Should Know:
- FAIR Health brief finds the total costs for all hospitalized COVID-19 patients range from a low of $362 billion in charges and $139 billion in estimated allowed amounts to a high of $1.449 trillion in charges and $558 billion in estimated allowed amounts, depending on the incidence rate of the infection in the US population.
- The total average charge per COVID-19 patient requiring an inpatient stay is $73,300 and the total average estimated allowed amount per
Read More
Scripps Launches App-Based Study Using Wearable Data to Predict Virus Outbreaks
What You Should Know:
- The Scripps Research Translational Institute launched DETECT, an app-based research study that will analyze wearable data shared by users with the goal of being able to more quickly detect fast-spreading viral illnesses, like flu and coronavirus.
- Early detection is critical for effective public health response to infectious disease outbreaks and for improving treatments. DETECT connects with smartwatches and activity trackers, including Fitbit devices,
Read More
New COVID-19 Community Vulnerability Map Uses Social Determinants of Health to Identify Populations At Greater Risk
What You Should Know:
- Jvion Launches COVID-19 Community Vulnerability Map to identify the social determinants of health (SDOH) that put populations at greater risk, informing community planning and resource allocation to proactively mitigate the risk to vulnerable populations.
- The Community Vulnerability Map allows users to search and drill down into communities to view populations most vulnerable for severe outcomes if infected with a COVID-like virus and the socioeconomic
Read More
Amazon, Epic, Mayo Clinic, Intermountain, Others Form COVID-19 Healthcare Coalition
What You Should Know:
The COVID-19 Healthcare Coalition is a
collaborative private-industry response to the novel coronavirus.
Its mission is to save lives by providing real-time
learning to preserve healthcare delivery and protect U.S. populations.
Coalition partners include Arcadia.io, athenahealth, Buoy
Health, the CommonWell Health Alliance, Epic, HCA Healthcare, Intermountain
Healthcare, LabCorp, Leavitt Partners, MassChallenge, Mayo Clinic, Microsoft,
MITRE, Rush
Read More
At-Home Respiratory Coronavirus Test Now Available to Texas Residents
What You Should Know:
- Wheel and imaware launch the first and only clinician-administered home-based COVID-19 lab testing in the nation, priced at $135.
- A trained healthcare professional will collect the sample at the patient's home to reduce the risk of false negatives associated with self-swabbing.
- CDC-authorized home-based tests can be delivered the same day, then Wheel clinicians trained in COVID-19 protocols are in contact with patients who test positive to
Read More
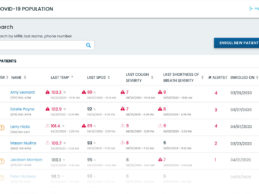
Validic Launches Real-Time, COVID-19 Remote Symptom Monitoring Tool
What You Should Know:
- Validic launches COVID-19 rapid deployment remote monitoring tool for employers and healthcare organizations to monitor employees, patients, and other individuals at scale for emerging symptoms of COVID-19.
- COVID-19 Home Monitoring tracks a person’s body temperature, difficulty breathing, cough frequency, and oxygen saturation.
- Triggered alerts will notify program administrators or clinicians as a person’s symptoms worsen, improve, or remain static – or as
Read More