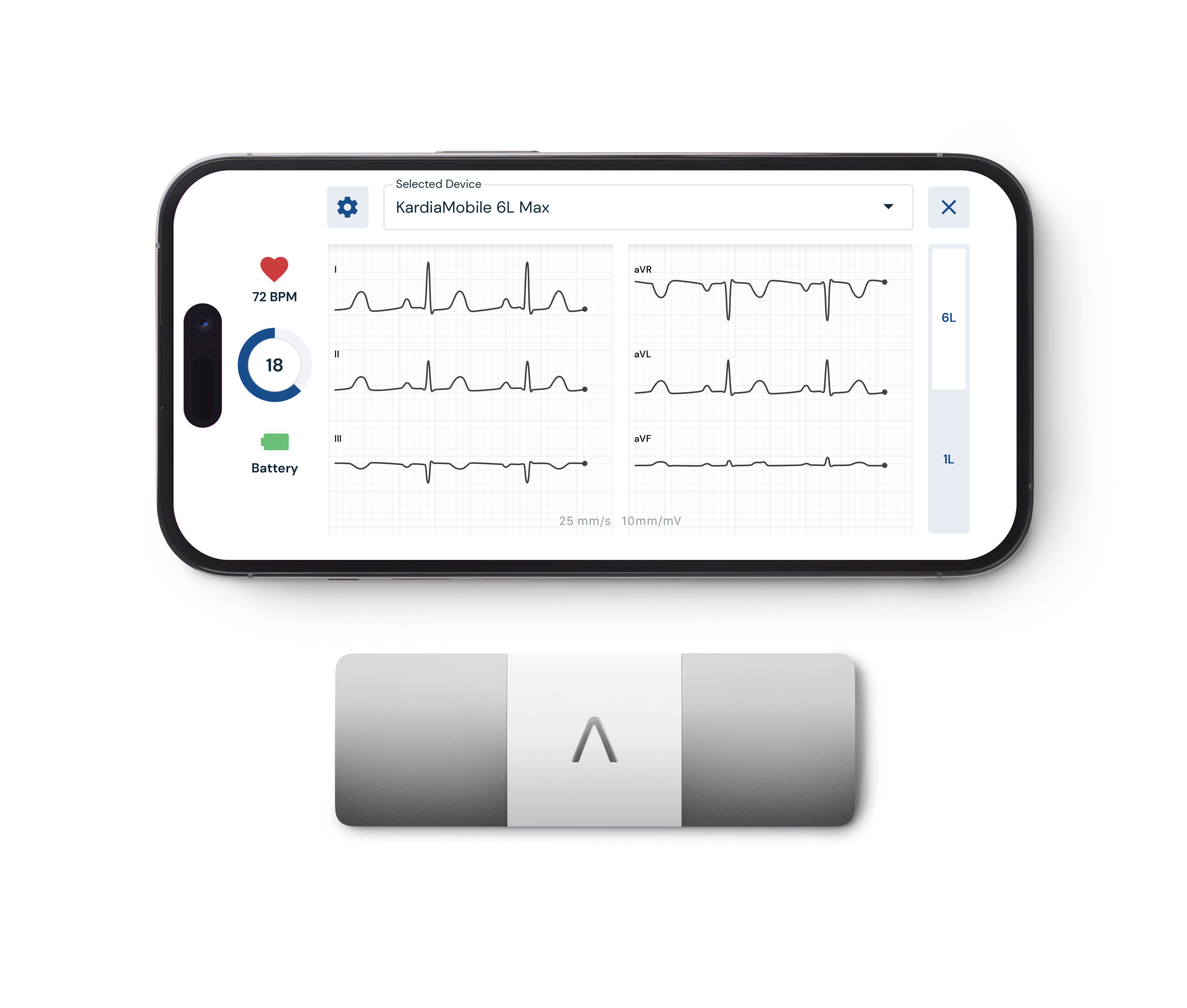
What You Should Know:
- AliveCor, a leader in FDA-cleared personal electrocardiogram (ECG) technology, today announced the launch of its most advanced personal ECG system, the AI-powered KardiaMobile® 6L Max.
- Accompanying this device is KardiaAlert™, a first-of-its-kind feature available exclusively to subscribers of AliveCor's KardiaCare service. Together, these innovations aim to provide users with more comprehensive detections and insights, monitor heart rhythms for
Read More
KardiaBand
ITC Judge Finds Apple Infringed AliveCor Patents
What You Should Know:
- Today, an International Trade Commission (ITC) judge issued an Initial Determination that Apple infringed AliveCor’s valid patented technology – a finding that may result in a limited exclusion order that will prohibit the import of infringing Apple Watches into the United States.
- AliveCor initially filed its complaint with the ITC in April 2021, alleging that Apple infringed AliveCor’s
Read More
AliveCor Receives “Breakthrough Device” Designation from FDA for Bloodless Hyperkalemia Test
AliveCor, a Mountain View, CA-based digital health startup developing solutions to transform cardiac care, announced on Monday received “breakthrough device” designation from the U.S. Food and Drug Administration for their bloodless hyperkalemia test. Developed with Mayo Clinic, AliveCor’s KardiaK Platform screens for elevated levels of blood potassium – hyperkalemia, without requiring any blood from the patient.This designation signals that the FDA will consider the technology on an
Read More
FDA Clears AliveCor’s KardiaBand for Apple Watch, Records Medical-Grade EKG in 30 Seconds
AliveCor, a FDA-cleared personal electrocardiogram (EKG) technology, today announced FDA clearance of KardiaBand in the U.S., allowing Apple Watch users to discreetly capture their EKG anytime, anywhere in order to quickly detect normal sinus heart rhythms and atrial fibrillation (AFib), the most common heart arrhythmia. The announcement marks first FDA-cleared medical device accessory for the Apple Watch, KardiaBand can record an EKG in 30 seconds with just a touch of its integrated sensor.
Read More