What You Should Know:
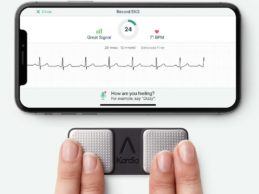
- AliveCor announced they received FDA clearance of new
algorithms for use with their personal EKG devices, KardiaMobile and
KardiaMobile 6L. These additional determinations will be available via a
software upgrade for the Kardia devices in 2021.
- The additional FDA-cleared algorithms double the number
of heart rhythm disturbances that AliveCor’s Kardia devices can detect,
broadening the number of patients who are able to use their remote
Read More
ecg
Eko Lands $65M to Expand AI-Powered Telehealth Platform for Virtual Pulmonary and Cardiac Exam
What You Should Know:
- Cardiopulmonary digital health company Eko raises $65M
in Series C funding to close the gap between virtual and in-person heart and
lung care.
- The latest round of funding will enable Eko to expand
in-clinic use of its platform of telehealth and AI algorithms for disease
screening and to launch a monitoring program for cardiopulmonary patients at
home.
Eko, a
cardiopulmonary digital
health company,
today announced $65 million in Series C funding led by
Read More
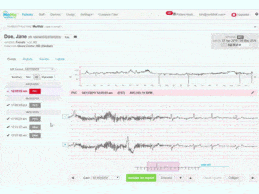
AI Leads Way to Less False Positives on Remote Cardiac Monitoring Devices, Improved Results
What You Should Know:
- Cardiac patients and their cardiologists are
experiencing a high number of false positives with remote patient monitoring
devices as a result of signal artifact providing inaccurate data, which can
lead to many complications—other than medical, such as unnecessary tests and
increased medical costs.
- Ambulatory cardiac monitoring provider InfoBionic has devised a way to decrease false positives and increase efficiency.
Remote cardiac monitoring’s false
Read More
Fitbit Awarded Regulatory Clearance in U.S. and Europe for ECG App to Identify AFib
What You Should Know:
- Fitbit has received 510(k) clearance from the
FDA, as well as CE marking in the European Union, for its ECG
app to assess heart rhythm for atrial fibrillation. The Fitbit ECG App is a
simple way people can take an on-the-spot reading of their heart rhythm at any
time, including whenever they notice any unusual cardiac symptoms.
- Fitbit Sense is the company’s first device compatible
with an ECG app that enables users to take a spot
Read More
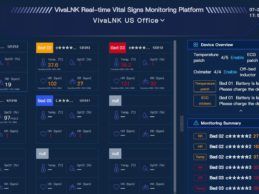
Multi-Vital Ambulatory Monitor for COVID-19 Patients Launches in the U.S.
What You Should Know:
- VivaLink launches a new multi-vital ambulatory monitoring solution for infectious respiratory diseases is being debuted in America and Western Europe. This monitoring solution is currently deployed in Asia, Eastern Europe, and the Middle East to help monitor COVID-19 patients.
- Built upon VivaLink’s proprietary ambulatory medical wearable sensor platform, the solution can easily be set up and scaled in hospitals, nursing homes, and remote environments. Even pop-up
Read More
AstraZeneca, Eko Collaborate to Advance Innovation Around Heart Failure
What You Should Know:
- Eko today announced a global collaboration with
AstraZeneca to accelerate the development of digital health tools for the
earlier screening of cardiovascular diseases, including heart failure.
- Through the collaboration, AstraZeneca and Eko will explore accelerating the development of Eko algorithms, enhancing clinical trials with Eko technology, and potentially building new heart failure detection solutions.
Eko, a digital health company
Read More
Philips Launches Pre-Hospital Wireless Monitoring Solution for Emergency Medical Response
What You Should Know:
- Philips receives 510(k) clearance from the FDA for its pre-hospital
wireless monitoring solution (Tempus LS- Manual), now offering its remote
monitor and defibrillator solution (Tempus ALS) to EMS customers in the U.S.
- Solution delivers real-time bidirectional data transfer
for remote patient monitoring, giving EMS responders a new approach to
pre-hospital care.
Philips, today announced the launch of its
remote monitoring and defibrillator solution
Read More
Eko Awarded $2.7M NIH Grant for Heart Murmur & Valvular Heart Disease Detection Algorithms
What You Should Know:
- The National Institutes of Health (NIH) has granted next-generation
cardiac AI company Eko an award totaling $2.7 million to support continued
collaborative work with Northwestern Medicine Bluhm Cardiovascular Institute
- The grant will focus on validating algorithms and help
more accurately screen for heart murmurs and valvular heart disease during
routine office visits with Northwestern Medicine.
- By incorporating data from tens of thousands of
Read More
Philips Unveils Fetal and Maternal Pod and Patch for Continuous, Non-Invasive Monitoring
What You Should Know:
- Philips unveils Avalon CL Fetal and Maternal Pod and Patch aim to reduce unnecessary physical interactions between clinicians and patients, which is of particular importance during the COVID-19 pandemic.
- The Fetal and Maternal Pod and Patch allow for continuous, non-invasive monitoring of maternal heart rate, fetal heart rate, and uterine activity with a single-use, 48-hour, disposable electrode patch placed on the mother’s abdomen.
Royal Philips,
Read More
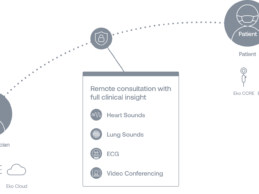
Eko Launches AI-Powered Telehealth Platform for Virtual Pulmonary and Cardiac Exam
What You Should Know:
Eko launches first AI-Powered telehealth platform for virtual
pulmonary and cardiac exams, providing clinicians with in-person level exam capabilities
during video visits.
Already deployed by more than 200 health systems for telehealth, platform goes beyond standard video conferencing to facilitate stethoscope audio, ECG live-streaming, and FDA-cleared identification of atrial fibrillation (AFib) and heart murmurs.
Eko today
announced Eko Telehealth, an
Read More