The fundamental problem with healthcare can be summed up in one sentence: We expect healthcare services that cater to our individual needs, yet the health care system operates under a one-size-fits-all, trial-and-error model. It is a model that results in missed diagnoses, protracted illnesses, and even premature death and wastes $935 billion annually.
The financial toll of this outmoded approach pales in comparison to the human toll. More than 128,000 people in the U.S. die each year
Read More
diagnostic testing
Boston Scientific Acquires Remote Cardiac Monitoring Company Preventice Solutions for $1.2B
What You Should Know:
- Boston Scientific announces an agreement to acquire Preventice Solutions, a leading developer of mobile health solutions and remote monitoring services that connect patients and caregivers for $1.2B.
- The acquisition of external cardiac monitoring technologies and services providers will expand Boston Scientific’s rhythm management diagnostics portfolio and capabilities.
Boston
Scientific today announced that it has acquired
Preventice Solutions, Inc.,
a
Read More
Mayo Clinic Launches On-Demand Diagnostic Testing Platform for COVID-19
What You Should Know:
- Mayo Clinic and Safe Health Systems launch a new health and connected diagnostics platform to lower costs and streamline access to care.
- The SAFE platform enables the rapid implementation of custom digital health applications, which combine digital provider services, artificial intelligence-based care automation, and remote point of care diagnostics.
Mayo Clinic and
Safe Health Group, Inc. announced the formation of Safe Health Systems, Inc.,
a
Read More
Talis Awarded $25M NIH Contract for Point-of-Care COVID-19 Testing
What You Should Know:
- Today, Talis Biomedical Corporation announced it has
been awarded a $25M contract from the National Institutes of Health (NIH) as
part of Phase 2 of its Rapid Acceleration of Diagnostics (RADx) initiative as
well as another $100 million in additional financing.
- The new funding will be used to scale manufacturing for
the launch of the Talis One™ diagnostic platform that provides rapid and highly
accurate detection of COVID-19 at points-of-care in 30 minutes
Read More
FDA Approves Cue Health’s Point-of-Care COVID-19 Test Using Nasal Swabs
What You Should Know:
- The Food and Drug Administration (FDA) has awarded Cue Health Emergency Use Authorization (EUA) for its rapid, portable, molecular point of care COVID-19 test.
- The company recently raised $100M in Series C funding from Menlo Park-based Decheng Capital, Foresite Capital, Madrone Capital Partners, Johnson & Johnson Innovation – JJDC, Inc., ACME Capital, and other investment firms.
Cue Health Inc.
(“Cue”), a healthcare
technology company,
Read More
FDA Approves Everlywell’s COVID-19 Test Home Collection Kit Using Nasal Swabs
What You Should Know:
- FDA approves Everlywell’s COVID-19 Test Home Collection Kit under the emergency use authorization (EUA) allows an individual to self-collect a nasal sample at home using Everlywell’s authorized kit.
- The Everlywell home-collection kit is currently the only authorized COVID-19 at-home sample collection kit for use with multiple authorized COVID-19 diagnostic tests.
The U.S. Food and Drug
Administration (FDA) has issued emergency use authorization (EUA) to
Read More
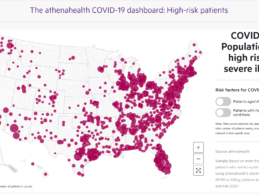
athenahealth Launches COVID-19 Dashboards Tracking Lab Test Orders & High-Risk Patients
What You Should Know:
- athenahealth launches two real-time COVID-19 dashboards for the healthcare community that enable users to track and predict which hospitals may need more support based on the number of tests being performed and the number of people at risk of developing severe illness from COVID-19.
- athenahealth’s COVID-19 high-risk populations dashboard identifies concentrated areas of high-risk patients, enabling local hospitals and clinicians to prepare for the
Read More
Amazon, Epic, Mayo Clinic, Intermountain, Others Form COVID-19 Healthcare Coalition
What You Should Know:
The COVID-19 Healthcare Coalition is a
collaborative private-industry response to the novel coronavirus.
Its mission is to save lives by providing real-time
learning to preserve healthcare delivery and protect U.S. populations.
Coalition partners include Arcadia.io, athenahealth, Buoy
Health, the CommonWell Health Alliance, Epic, HCA Healthcare, Intermountain
Healthcare, LabCorp, Leavitt Partners, MassChallenge, Mayo Clinic, Microsoft,
MITRE, Rush
Read More
At-Home COVID-19 Test Kits Will Be Available to U.S. Consumers for $139 on March 23rd
What You Should Know:
- Everlywell announces that an at-home collection kit with telehealth diagnosis for COVID-19 will be available to consumers starting Monday, March 23 for $139.
- The initial offering of 30,000 collection kits will significantly impact total coronavirus testing capacity in the United States; free telehealth consults are included for those with positive results.
- By working with multiple labs to scale infrastructure, the company plans to have testing and diagnosis
Read More
Lifepoint Informatics Launches App for DrChrono EHR Users to Manage Lab Reports
- Lifepoint leveraged DrChrono’s API to create an app that works seamlessly with its EHR platform to easily order and process lab reports and results. - Medical professionals can place their orders directly within DrChrono EHR via Lifepoint’s app and it will transmit orders electronically.DrChrono Inc., the company enabling the medical practice of the future announced today a new partnership with Lifepoint Informatics, a leader in healthcare IT focusing on laboratory outreach connectivity,
Read More