What You Should Know:
- Immunai, a leading AI biotech company specializing in mapping the human immune system and the Parker Institute for Cancer Immunotherapy (PICI), a collaborative consortium of the world’s leading immuno-oncology experts, announced today that they will build the world’s largest single-cell dataset from patients treated with standard-of-care (SoC) immunotherapy.
- Under the collaboration with PICI,
Read More
Life Sciences | News, Analysis, Insights - HIT Consultant
DHL Express Launches Next-Day Brazil-to-USA Medical Express Service for Clinical Trials
What You Should Know:
- DHL Express, the world's leading international express services provider, today announced a significant expansion of its specialized Medical Express (WMX) service, adding a next-day delivery capability for the critical Brazil-to-USA lane.
- The enhancement connects South America and Puerto Rico more efficiently to the United States, providing crucial support for customers in the pharmaceutical and clinical research sectors navigating complex global
Read More
Eli Lilly’s Oral GLP-1 Shows Positive Trial Results for Type 2 Diabetes
What You Should Know:
- Eli Lilly and Company announced positive topline Phase 3 results from ACHIEVE-1, a study evaluating the safety and efficacy of orforglipron compared to placebo in adults with type 2 diabetes whose blood sugar was inadequately controlled through diet and exercise alone.
- Orforglipron stands out as the first oral small molecule glucagon-like peptide-1 (GLP-1) receptor agonist, taken without food and water restrictions, to successfully complete a Phase 3
Read More
The AI Hype Cycle in MedTech: Navigating the Trough of Disillusionment
The emerging technologies hype cycle had multiple AI applications at the peak of inflated expectations in 2023 and were just beginning the ride down to the Trough of Disillusionment in 20241 2. For years, it’s been said that “the use of data and algorithms is going to transform healthcare.” While true to a degree, we see that MedTech companies that invest in trends like artificial neural networks, analytics, big data analytics, and now AI, often struggle to yield a significant return on
Read More
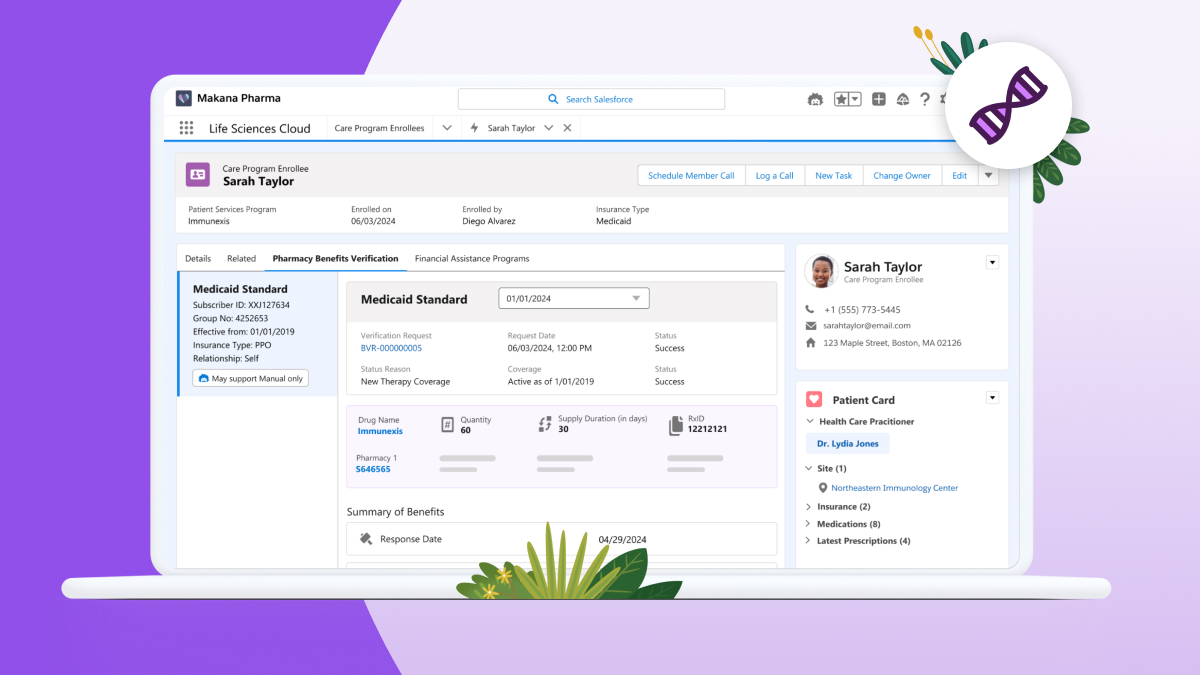
Sware & Salesforce Partner to Bring AI-Driven Technology to the Life Sciences Cloud
What You Should Know:
- Sware, the leading provider of comprehensive GxP validation solutions for innovative life sciences and technology companies, today announced that it will deliver GxP computer systems validation of Salesforce Life Sciences Cloud.
- Life Sciences Cloud empowers pharmaceutical and medical technology organizations to accelerate drug and device development, recruit and retain patients across clinical trials, and leverage AI to deliver personalized customer
Read More
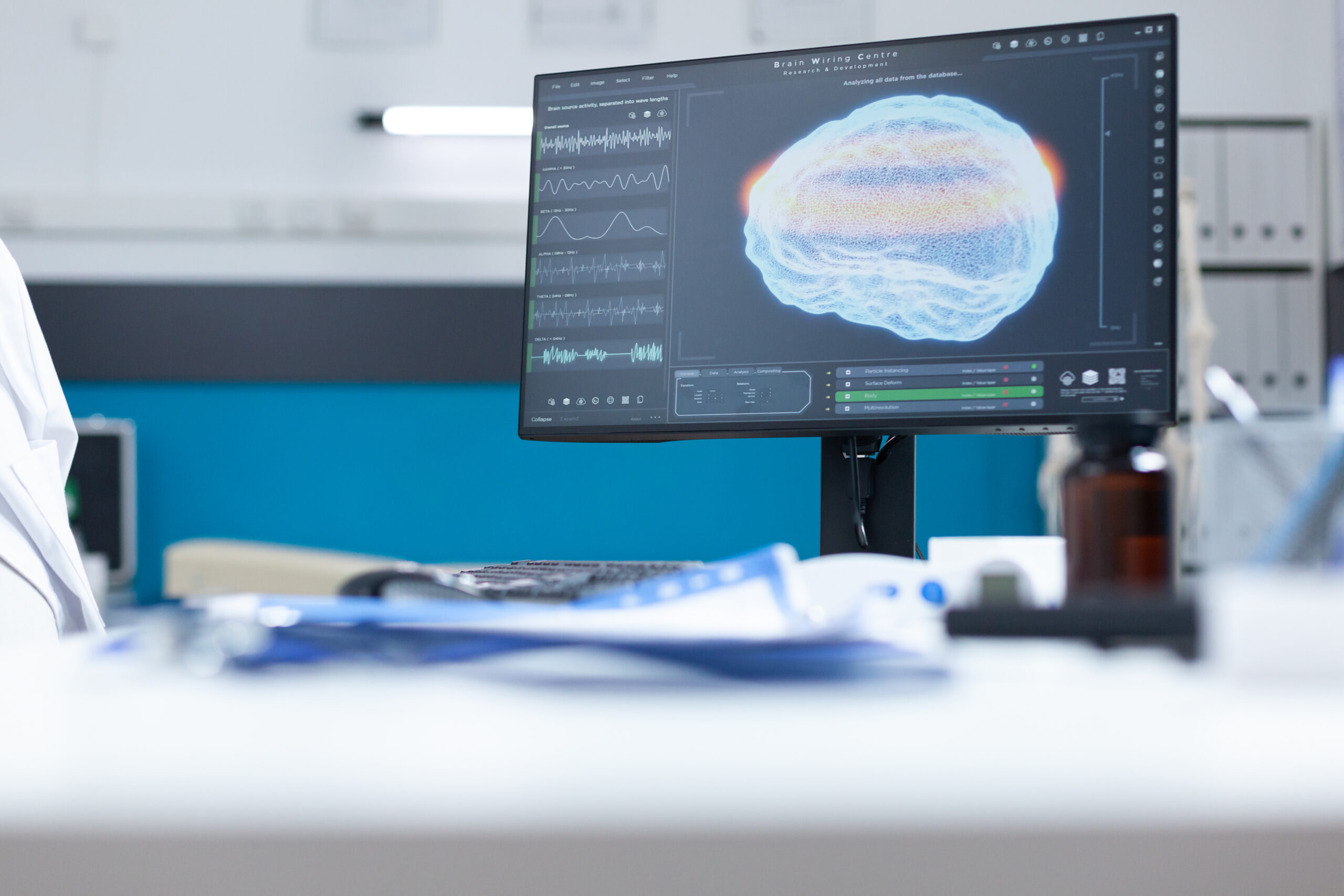
How Are Advances in Neurologic Technology Changing Diagnosis and Treatment?
From sophisticated imaging techniques to gene therapies and artificial intelligence, the landscape of neurological care is evolving at an unprecedented pace. These innovations are revolutionizing how we diagnose and treat neurological disorders, offering hope for improved outcomes and a better quality of life for millions affected by conditions impacting the brain, spinal cord, and peripheral nerves.
Advanced Neuroimaging
One of the most significant areas of progress lies in
Read More
illumicell AI Raises $2M to Transform Semen Diagnostics with Real-Time AI
What You Should Know:
- Techmed startup illumicell AI, founded by a team of Harvard-trained physician Michel Bielecki, former McKinsey consultant Jeyla Sadikova, and rocket engineer Loup Cordey, has secured $2M in pre-seed funding. The funding round was supported by a combination of healthcare operators, clinicians, and early-stage funds, including life science investors KOFA Healthcare, Harvard Phoenix Venture Fund, and MedTechSyndicates
- The company aims to transform fluid-based
Read More
Flatiron Health and Massive Bio Partner to Improve Clinical Trial Enrollment
What You Should Know:
- Flatiron Health, a healthtech company focused on transforming clinical research through technology that integrates research into everyday care, has announced a strategic partnership with Massive Bio, a global leader in using artificial intelligence to streamline patient identification and recruitment for clinical trials.
- The partnership aims to expand patient identification capabilities and improve clinical trial enrollment across the U.S.
Enhance
Read More
Simplifying Clinical Trials: Addressing Technology Challenges for Site Teams
In recent years, clinical research and development stakeholders have put tremendous effort into improving patient-centered clinical trials, where patients feel valued as partners in the processes and approaches to care that impact their health. Clinical trial sponsors are keenly aware that, to do so, the use of advanced clinical technologies, including decentralized solutions, has been necessary to improve patient experience and accelerate drug development. But as sponsors implement multiple
Read More
How 3D Modeling is Transforming Medical Imaging and Diagnosis
In the 21st century, we are fortunate to benefit from perpetual advances in medicine. Researchers continue to discover new procedures and treatments, and those advances often rely on technology. Improvements in hardware, internet speeds, interconnected devices, and artificial intelligence all contribute to higher efficiency, personalization, and accessibility in treatments.
Technologies such as advanced imaging and modeling (AIM), augmented reality/virtual reality (AR/VR), 3D printers,
Read More