
Zebra Medical Vision, an Israli-based deep learning imaging analytics company, today announces that it has received FDA 510(k) clearance for HealthPNX – an AI-powered alert for pneumothorax (PNX), based on chest X-rays.
FDA 510(k) Clearance Details
Received in May 2019, the FDA 510(k) clearance focuses on an AI alert for “stat” (urgent) findings of pneumothorax and demonstrates a promising potential to substantially reduce turnaround time and increase the radiologist’s confidence in making this diagnosis. Chest X-ray is one of the world’s most used imaging modalities.
Impact of Pneumothorax
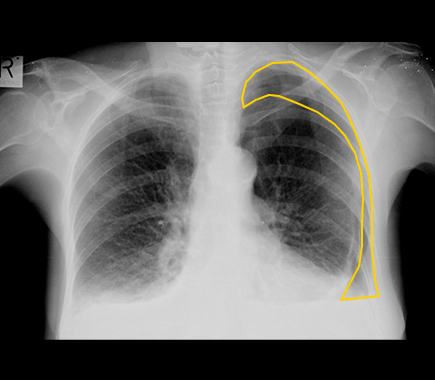
Pneumothorax is a condition in which there is an accumulation of gas within the pleural space between the lung and the chest wall. Without prompt management, pneumothorax can lead to total lung collapse and other potentially fatal complications.
This condition is most commonly diagnosed by a chest X-ray scan, though it is one of the hardest to interpret, and is known for high disagreement rates even between experienced radiologists. Misdiagnosis or late-diagnosis of Pneumothorax impacts around 74,000 Americans per year.
Zebra-Med’s All-In-One Solution
The patent-pending technology automatically detects findings suggestive of Pneumothorax based on CXR or digital radiography (DR) scans and alerts the medical team. In hospitals where Zebra-Med’s “All in one” (AI1) solution is integrated into the radiologist’s worklist, the scan is flagged so that radiologist can address it in a timely manner.
Impact of FDA 510(k) Clearance
This first of its kind FDA cleared solution can save physicians more than 80% of the time taken to reach the acute condition, compared to the traditional First In First Out (FIFO) methodology.
Clinical Validation Study
The Pneumotorax product is a result of the extensive work accomplished by Zebra-Med’s research lab. The chest X-ray AI network was trained using millions of images to identify over 40 common clinical findings. The results of the Textray study establish a new bar for AI research in medical imaging, demonstrating high rates of agreement between the algorithm and human radiologist experts.
“In a clinical validation study we performed, Zebra-Med’s acute CXR pneumothorax and CT Brain bleed products demonstrated a promising potential to substantially reduce turnaround time and increase the radiologist’s confidence in making these diagnoses,” said Dr. Terence Matalon, Chairman of Imaging at Albert Einstein Medical Center. Zebra-Med’s AI1 Triage Solution is the first of its kind for both CTs and X-rays, and currently addresses two acute conditions: intracranial hemorrhages (head CTs, FDA pending), and pneumothorax (chest X-rays).
