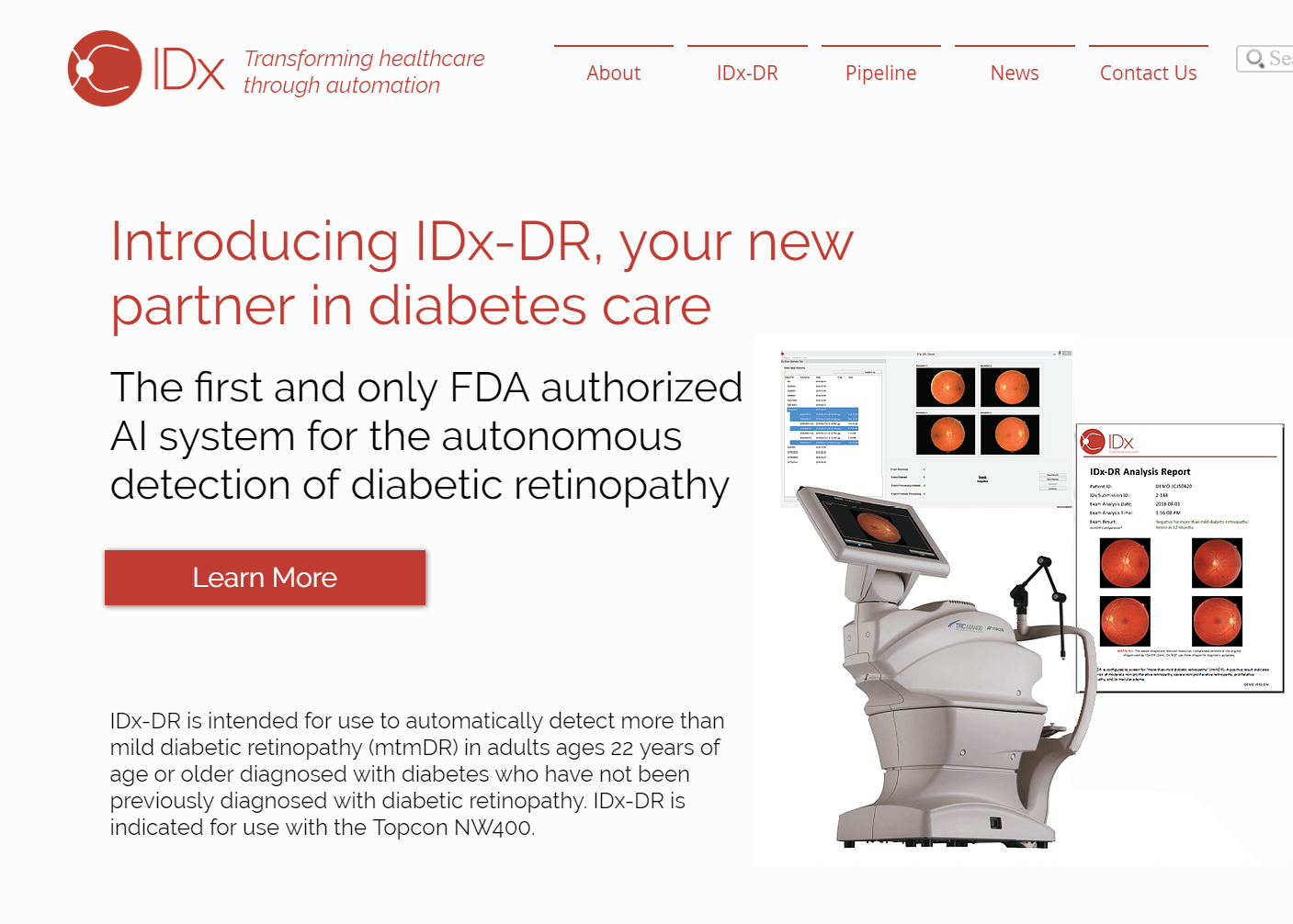
IDx, an AI diagnostics company, and Topcon, one of the world’s leading ophthalmic device manufacturers, have signed an exclusivity agreement that will allow the companies to scale delivery of AI-based diagnostic solutions in the U.S. market. As part of the agreement, is granted exclusive rights to IDx-DR system, an autonomous AI system that instantly detects diabetic retinopathy in fundus images, exclusively with Topcon NW400, an easy to use, robotic fundus camera.
IDx-DR is an FDA-cleared AI-based diagnostic system designed for use at the front lines of care to detect diabetic retinopathy. IDx-DR is intended for use by healthcare providers to automatically detect more than mild diabetic retinopathy in adults (22 years of age or older) diagnosed with diabetes who have not been previously diagnosed with diabetic retinopathy.
The recent FDA clearance of IDx-DR followed a clinical trial that demonstrated the autonomous AI system’s ability to safely deliver specialty-level diagnostics in primary care settings. Novice operators received minimal training on how to capture images using the Topcon NW400 and IDx-DR’s image quality AI. They successfully produced a diagnostic result 96% of the time by consistently capturing the high-quality images needed for IDx-DR to make a disease assessment.
“The importance of ease of use and consistently high image quality to successfully implement autonomous AI diagnostics in the front lines of care cannot be stressed enough,” said Michael D. Abramoff, MD, Ph.D., founder, and CEO of IDx. “Topcon is truly the innovative market leader in developing highly automated, consistent and easy to use retinal imaging systems. Together we can prevent visual loss and blindness by ensuring the safety, accessibility, and affordability of early disease detection.”
Both companies have a history of innovation. IDx received FDA clearance for IDx-DR in April 2018, marking the first time the FDA has cleared an autonomous AI diagnostic system that does not require a physician to interpret images or results. Topcon introduced the first commercial back-of-the-eye spectral domain (SD) and swept source (SS) optical coherence tomography (OCT) systems and more recently announced the widely successful Maestro and Triton OCTs.
Topcon recently announced the release and class II clearance of Topcon Harmony, a diagnostic data management application with vendor-neutral connectivity that enables software providers like IDx, EHRs, and third-party manufacturers to seamlessly integrate diagnostic results into one central location for eye care providers.
IDx is developing additional AI-based diagnostic systems for the detection of macular degeneration, glaucoma, Alzheimer’s disease, cardiovascular disease, and stroke risk. Prior to this current round, IDx had been funded by a group of private angel investors since the company’s founding in 2010.
