More than 4 million recalled medical devices may harm patients in 2021. And that’s just what we can see has been “posted” on the FDA site through June 25, 2021. The number of medical device recalls issued halfway through 2021 is far higher than in the previous five years. Healthcare facilities are now facing both a higher number of recalls and a shortage of essential medical devices and products.
The FDA has noted that recalled medical devices caused almost 100,000 deaths between 2008
Read More
Medical Devices | FDA | News, Analysis, Insights - HIT Consultant
FDA Clears World’s First and Only Smart Knee Implant
What You Should Know:
- Zimmer Biomet Holdings, a global medical technology leader, and Canary Medical, a medical data company, announced U.S. Food and Drug Administration (FDA) De Novo classification grant and authorization to market the tibial extension for Persona IQ®, the world's first and only smart knee cleared by the FDA for total knee replacement surgery.
- Persona IQ® combines Zimmer Biomet’s proven and trusted knee implant, Persona® The Personalized Knee®, with Canary Medical’s
Read More
MindRhythm Raises $5M for Stroke Triage Medical Device in EMS Settings
What You Should Know:
- MindRhythm, a medical technology company focused on preventing neurological injury in stroke, today announced it has raised $5 Million in an oversubscribed round of seed funding. Principal investors include Pierre and David Lamond; Corey Goodman, PhD, Managing Partner of venBio and Board Chairman for MindRhythm; DCVC; Aestus Capital; Perseverance Capital Management; Blue Fog Capital; Franklin Berger, Managing Director at FMB Research and Kyle York, CEO and managing
Read More
Talking about Cybersecurity Vulnerabilities in Medical Devices Shouldn’t be Taboo
According to the National Vulnerability Database, 18,353 vulnerabilities were reported in 2020. That’s nearly three times the volume of vulnerabilities reported five years ago, and higher than any year in the previous two decades. Given the rise in connected devices, this increase is not entirely unexpected. If that’s the case, shouldn’t we be seeing more vulnerability disclosures related to medical devices?
The U.S. Department of Homeland Security Cybersecurity and Infrastructure
Read More
Validic Integrates Medical Device Data with Wellframe App
What You Should Know:
- Today, Wellframe and Validic® announced a strategic collaboration today, empowering Wellframe members to integrate medical device data within the Wellframe mobile app. This integration with Validic will first enable Wellframe users to track their blood glucose levels across 50 glucometer devices, right from the Wellframe app. Wellframe’s ability to collect meaningful biometrics data from their digital care management platform allows for more touchpoints with care teams
Read More
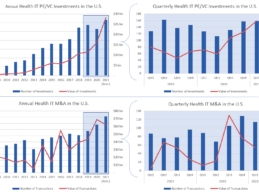
Q1 2021 Health IT/Digital Health PC/VE, M&A, IPOs/ SPACs Activity
The first quarter of 2021 has been one of investor optimism as the vaccine rollout continues ahead of expectations and economic activity begins to accelerate in response. Within the Health IT industry, the already strong investment and M&A trends seen in 2020 have only accelerated. Over the course of the quarter, we observed $7 billion in private equity and venture capital investment across 158 companies.
By comparison, Q1 of 2020 saw $3.2 billion and 127 investments. Health IT
Read More
BioIntelliSense Partners with Leukemia & Lymphoma Society for Continuous Monitoring in Clinical Trials
What You Should Know:
-BioIntelliSense announced it has entered into a strategic partnership with The Leukemia & Lymphoma Society (LLS) to incorporate the BioSticker™ platform in clinical trials of hematological cancer patients.
- The LLS clinical trials will include use of the BioSticker medical device for the continuous collection of vital sign and physiological data which includes temperature, heart rate, respiratory rate at rest, activity level and body position. The BioSticker
Read More
Life Sciences Firm OrbiMed Raises $3.5B Across Private Investment Funds
What You Should Know:
Life sciences investment firm OrbiMed announced $3.5 billion in commitments for its latest private investment funds, including $1.5 billion for OrbiMed Private Investments VIII, $800 million for OrbiMed Asia Partners IV, and $1.2 billion for OrbiMed Royalty & Credit Opportunities III.
The three new funds include a broad range of medical institutions, university endowments, foundations, pension funds and sovereign wealth funds.
- OrbiMed Private Investments
Read More
ECRI Institute: Top 10 Health Technology Hazards to Watch in 2021
What You Should Know:
- ECRI Institute identified the complexity of managing medical devices with COVID-19 emergency use authorization as the number one technology hazard in its Top 10 Health Technology Hazards for 2021 report.
ECRI Institute’s Top 10 Health Technology Hazards, now in its 14th year, identifies top health technology concerns that warrant attention from healthcare leaders. The hazards selected are based on a rigorous review of ECRI’s incident
Read More
Why Cancer Registries are Part of America’s Fight for Racial Equality
According to a 2019 study in the Proceedings of the National Academy of Sciences, one in 1,000 Black males can expect to die at the hands of the police. Black males were also 2.5 times more likely to die during an encounter with police than white males. For some, those figures are staggering. For many others, they are reminders of the racial disparities that have existed for far too long in this country.
So, in 2020, when recent events have brought racial injustice back into the national
Read More