What You Should Know:
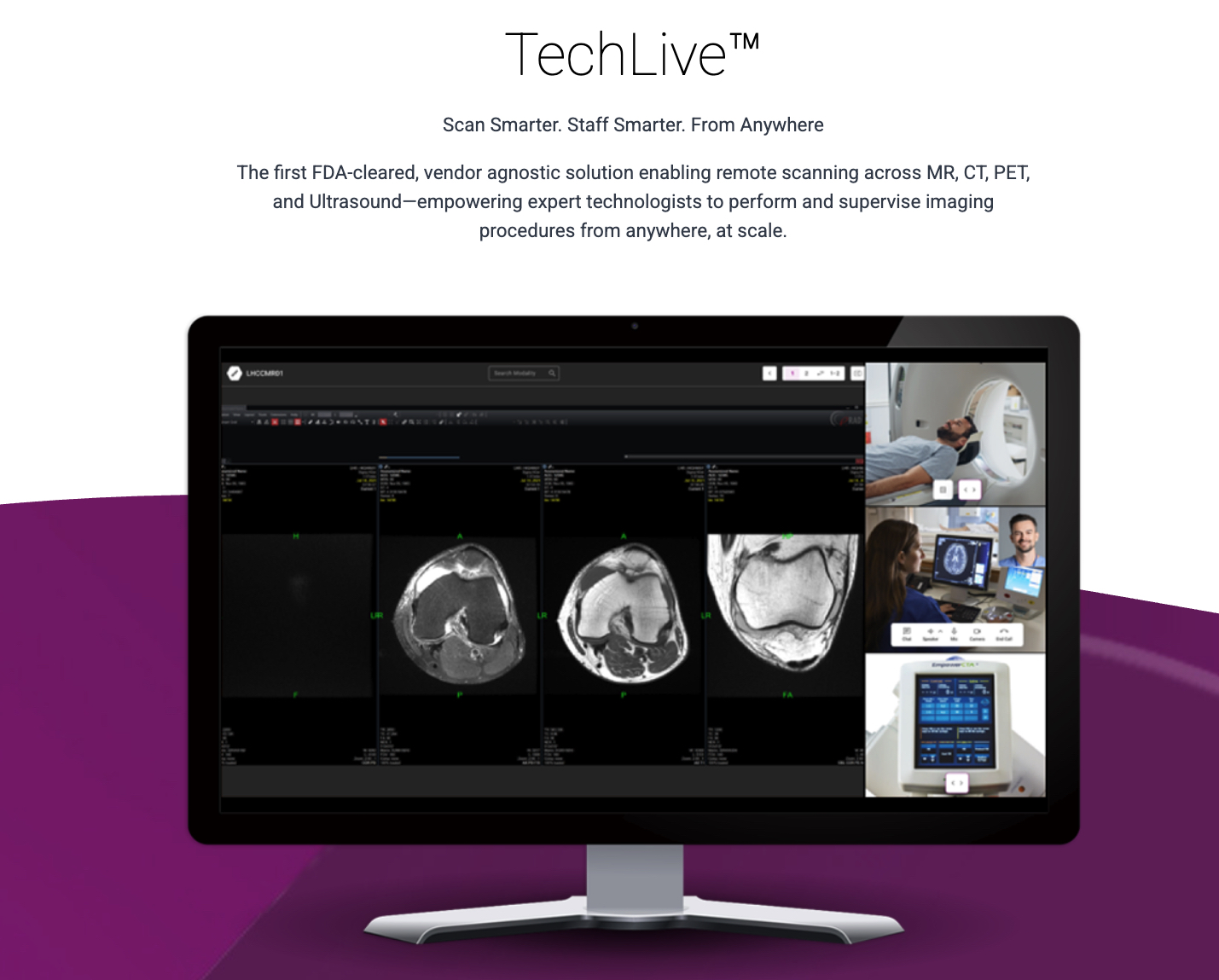
- DeepHealth, a subsidiary of RadNet, has received FDA 510(k) clearance for its new remote scanning solution, TechLive™. The vendor-agnostic platform is designed to address the growing radiology technologist shortage by allowing experts to remotely operate and supervise MR, CT, PET/CT, and Ultrasound procedures from a centralized command center.
- The move comes at a critical time for the healthcare industry, which has seen radiology workloads steadily increase
Read More
FDA Clearance
Build Smart, Not Fast: What the FDA Really Cares About in Digital Therapeutics
Digital therapeutics (DTx) are revolutionizing healthcare — from smart inhalers to virtual therapy avatars. As more patients turn to DTx apps, companies rush to deliver innovative features. But in the race to innovate, many developers neglect vital elements of success, leading to major setbacks, especially when seeking FDA approval. It’s time to find a practical path forward to mitigate risk and scale smarter.
The Digital Therapeutics Trifecta:
Read More
PathAI Granted FDA Clearance for AISight Dx for Primary Diagnosis in Clinical Settings
What You Should Know:
- PathAI, a global leader in artificial intelligence (AI) and digital pathology solutions, today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its digital pathology image management system, AISight® Dx, for use in primary diagnosis in clinical settings.
- The FDA clearance builds upon the platform's initial 510(k) clearance for AISight Dx(Novo) in 2022, demonstrating PathAI’s ongoing commitment to innovation and
Read More
FDA Approves Drug-Free AI-Powered Wearable for Nasal Congestion for Kids
What You Should Know:
- SoundHealth, a medical technology company leveraging artificial intelligence and medical science, recently announced it has received U.S. Food and Drug Administration (FDA) approval for pediatric use of its SONU Band.
- The AI-enabled wearable device is now cleared as the first FDA-approved, drug-free solution for treating moderate to severe nasal congestion due to allergic and non-allergic rhinitis in children aged 12 and up, offering a safe alternative
Read More
Arineta’s SpotLight Duo CT Scanner Receives FDA Clearance for Low-Dose Lung Cancer Screening
What You Should Know:
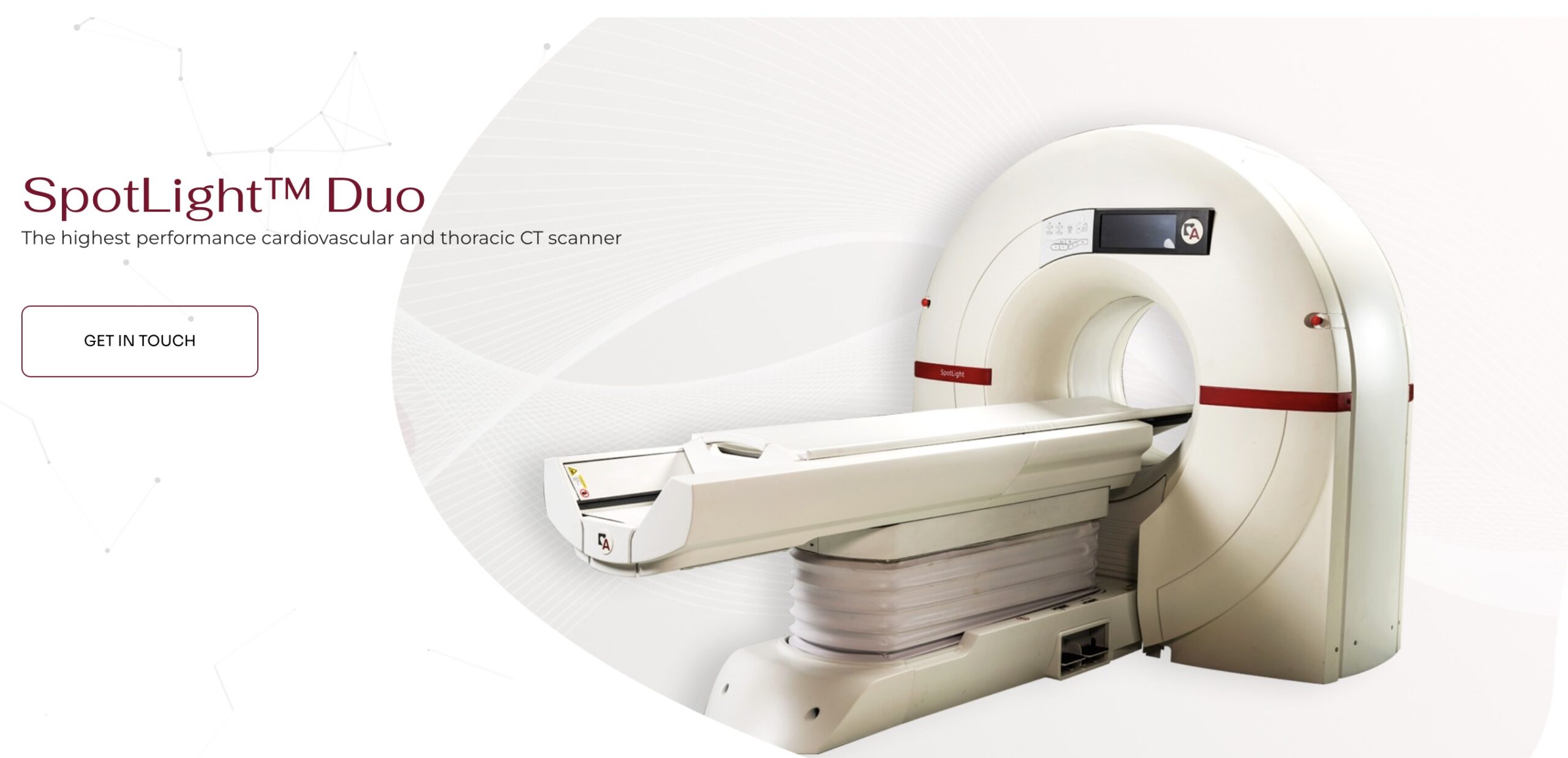
- Arineta, a leader in advancing cardiovascular imaging solutions, announced that its SpotLight™ Duo cardiac CT scanner has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for low-dose lung cancer screening (LDCT).
- The regulatory milestone allows healthcare providers to utilize a single, ultra-fast CT platform for both comprehensive cardiac imaging and vital lung cancer screening, offering more complete and efficient care for
Read More
Osteoboost Launches First FDA-Cleared Prescription Wearable Nationwide to Combat Low Bone Density
What You Should Know:
- Osteoboost Health announced the nationwide availability of Osteoboost, a groundbreaking prescription medical device. Osteoboost aims to provide hope for millions diagnosed with low bone density, helping to reduce the risk of fractures that can severely impact mobility and independence.
- As the first and only FDA-cleared device of its kind for low bone density, Osteoboost introduces a novel, protective, and preventative approach to bone health, offering a new
Read More
World’s First AI Lung Sound Suite: TytoCare Gets FDA Clearance for Rhonchi
What You Should Know:
- TytoCare, a pioneering virtual care company dedicated to delivering accessible, high-quality primary care from the comfort of home announced the company has become the first in the world to receive FDA clearance for its AI-based detection of all three major abnormal lung sounds.
- The milestone was reached with the FDA clearance of Tyto Insights™ for Rhonchi Detection, completing TytoCare’s comprehensive AI-powered lung sound suite. With this latest
Read More
Click Therapeutics Obtains FDA Authorization for First Prescription Digital Therapeutic for Migraine Prevention
What You Should Know:
- Click Therapeutics, Inc. (“Click”), a leader in the rapidly evolving field of prescription medical treatments, encompassing both prescription digital therapeutics (PDTs) and software-enhanced drug™ therapies has received FDA marketing authorization for CT-132, the first prescription digital therapeutic specifically indicated for the preventive treatment of episodic migraine in adults aged 18 and older.
- The FDA granted a De Novo Classification Request for
Read More
Tandem Diabetes Care Receives FDA Clearance for Control-IQ+ Technology for Type 2 Diabetes
What You Should Know:
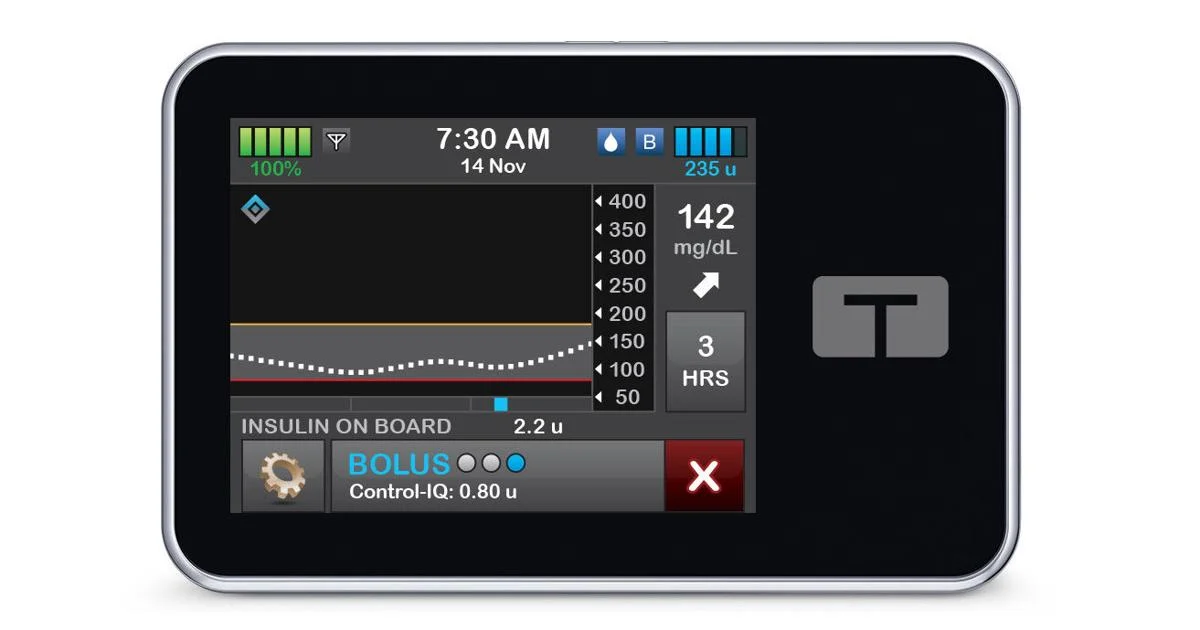
- Tandem Diabetes Care, a insulin delivery and diabetes technology company, announced that its Control-IQ+ technology has received clearance from the U.S. Food and Drug Administration (FDA) for use by adults with type 2 diabetes.
- The expanded indication builds on the success of Control-IQ+ in type 1 diabetes and offers a new automated insulin delivery (AID) option for individuals with type 2 diabetes.
Improve Glycemic Control for Adults with Type 2
Read More
Ezra Receives FDA Clearance for Enhanced AI-Powered MRI Technology
What You Should Know:
- Ezra, a innovator in AI-driven cancer screening, today announced that its advanced Ezra Flash AI model has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
- The FDA clearance expands the capabilities of its AI-powered platform to provide faster, more affordable, and higher-quality full-body MRI scans.
Ezra Flash AI for MRI Image Quality
Ezra Flash, a Class II medical device, now enhances image quality for neuro, abdomen,
Read More