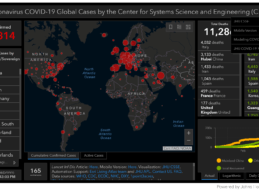
What You Should Know:
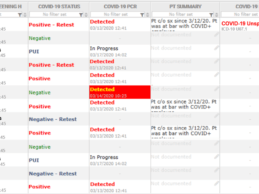
- TransformativeMed offers free COVID-19 tracking and workflow solution to Seattle-based hospitals and medical centers during the COVID-19 pandemic.
- The application works with electronic health records to track patient medical tests; helps with coronavirus patient workflows.
- COVID-19/CORES is already being used at UW Medicine, which includes the University of Washington Medical Center and Harborview Medical Center.
TransformativeMed, which transforms
Read More
Coronavirus (COVID-19)
Microsoft, Adaptive, Providence Partner to Decode COVID-19 Immune Response
What You Should Know:
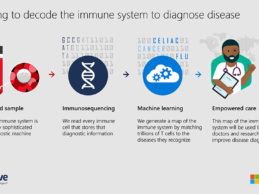
- Microsoft and Adaptive Biotechnologies have expanded their partnership to map the human immune response to COVID-19 and advance our understanding of the disease.
- Data from this study will be made available through an open-access portal to help researchers, public health organizations and industry improve diagnostics, help triage patients based on the immune response to the disease, and inform vaccine discovery for COVID-19.
- Other industry leaders including
Read More
New Website Helps People Find Nearby COVID-19 Testing Centers Nationwide
What You Should Know:
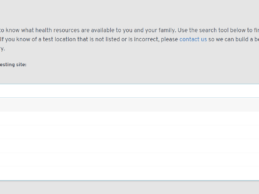
- Chicago-based technology company Evive launched the Evive.Care website, which is helping people find COVID-19 (Coronavirus) testing centers near them.
- The Evive.Care search tool allows people to enter their state and county and then displays a list of nearby COVID-19 testing centers.
- There are currently 240+ testing centers on the website across approximately 200 counties in the U.S.—those numbers are constantly evolving.
Evive, a Chicago,
IL-based
Read More
Scanwell Health Launches Clinical-Grade Rapid At-Home COVID-19 Test Kits
What You Should Know:
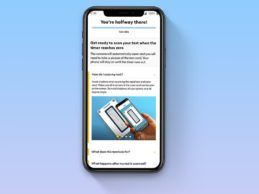
- Scanwell Health announced the first clinical-grade rapid at-home test for COVID-19.
- Scanwell secures exclusive rights to license China FDA-cleared rapid serology test from INNOVITA; Partners with nationwide telehealth provider Lemonaid Health to distribute smartphone-enabled tests to individuals in the U.S.
- After a quick screening, eligible patients are sent a kit via next-business-day delivery. Patients take the test at home and
Read More
AWS Launches $20M Initiative to Accelerate COVID-19 Diagnostics, Research, and Testing
What You Should Know:
- Amazon Web Services (AWS) announced its commitment of $20 million and the launch of the Diagnostic Development Initiative to support customers who are working to bring better, more accurate, diagnostics solutions to market faster and promote better collaboration across organizations.
- With the assistance of AWS, customers can accelerate diagnostic research, innovation, and development to speed our collective understanding and detection of COVID-19 and other
Read More
Sharecare Launches Comprehensive, Medically Verified COVID-19 Information Hub
What You Should Know:
- Sharecare announced the availability of a central COVID-19 information hub featuring the latest developments, guidance, and resources in response to the pandemic.
- The Sharecare COVID-19 information hub was created to provide people with easy access to the latest content and tools for COVID-19 preparedness, awareness, and prevention including interactive maps and data; articles, videos, and infographics detailing the latest news – in addition to direct links to
Read More
COVID-19’s Impact on Telehealth & HIPAA Regulations
The declaration of COVID19 as a pandemic and the United States declaring a national emergency to help contain and enhance treatment access have resulted in a number of changes to ease potential regulatory burdens or barriers in healthcare. Like so much happening as a result of the pandemic, the changes have the potential to fundamentally change the healthcare industry not just during the time of the emergency declaration.
Of particular note are coordinated announcements from various agencies
Read More
At-Home COVID-19 Test Kits Will Be Available to U.S. Consumers for $139 on March 23rd
What You Should Know:
- Everlywell announces that an at-home collection kit with telehealth diagnosis for COVID-19 will be available to consumers starting Monday, March 23 for $139.
- The initial offering of 30,000 collection kits will significantly impact total coronavirus testing capacity in the United States; free telehealth consults are included for those with positive results.
- By working with multiple labs to scale infrastructure, the company plans to have testing and diagnosis
Read More
COVID-19: Glooko Offers Free Remote Care to Medical Clinics and People with Diabetes
What You Should Know:
- Glooko today announced that during the COVID-19 pandemic they are taking action to serve the global diabetes community by providing a no-charge remote care solution that provides live patient-to-clinician connectivity.
- To minimize the risk to people with diabetes during this time by broadening access to a remote medical appointment with healthcare providers, Glooko is offering its secure, privacy-protected remote care solution at no charge to medical clinics and
Read More
Jump Technologies Offers Hospitals Free Supply Chain Solution for Tracking COVID-19 Supplies
What You Should Know:
- Jump Technologies offers new supply chain solution free of charge to help hospitals manage utilization of critical supplies and avoid stockouts during high volume COVID-19 response.
- From a single screen, hospitals can easily see where existing supplies are located (in hospital storage or in a warehouse); track consumption by department, clinic, or user; and forecast potential stockouts.
The rapid spread of the novel coronavirus (COVID-19) has placed unique
Read More