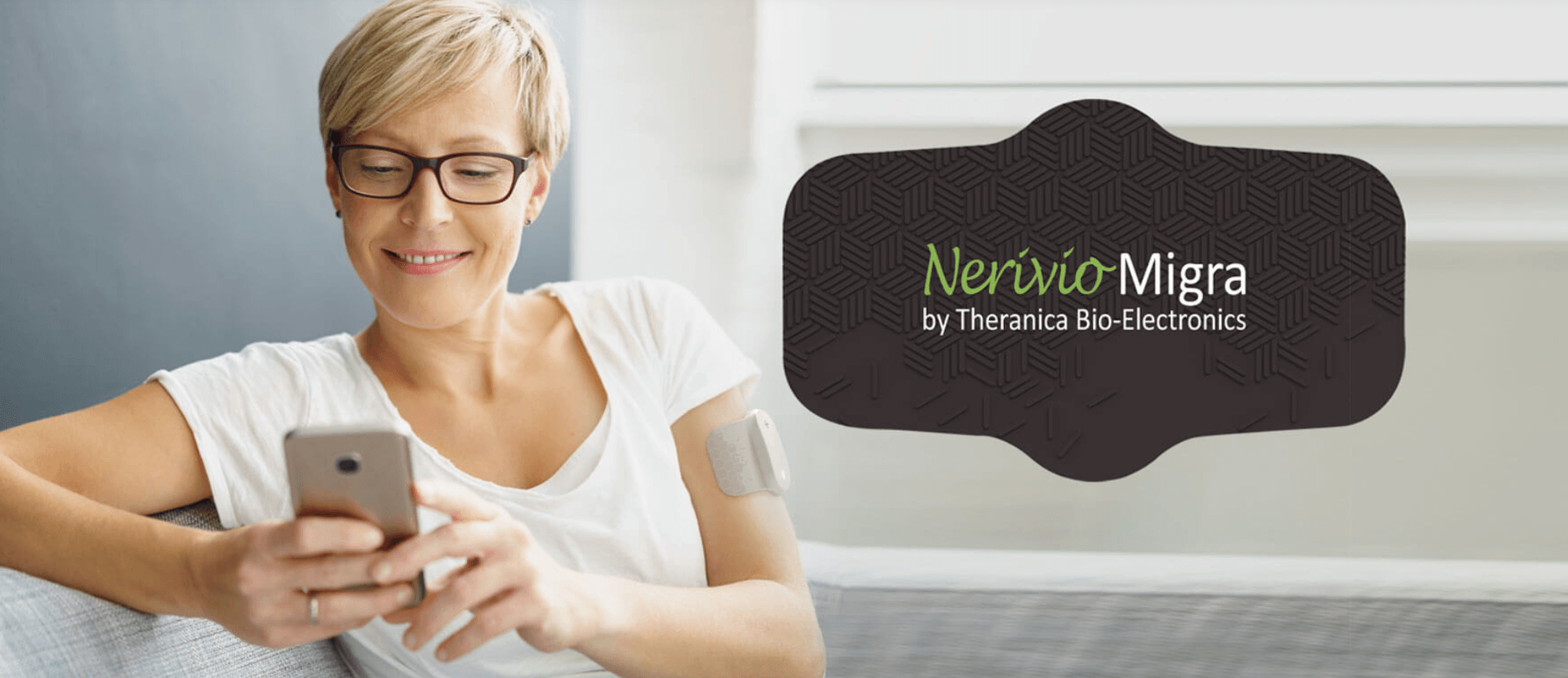
Today, Theranica announced that the FDA has granted De Novo clearance for Nerivio Migra, the first-ever smartphone controlled wearable for the acute treatment of migraines. Worn on the upper arm, Nerivio Migra utilizes remote electrical neuromodulation for the acute treatment of migraine that is proven to be as effective as leading devices and drugs on the market in treating acute migraine, with fewer and more mild side effects.
Migraine is the third most common disease in the world, with an estimated global prevalence of 14.7% of the world’s population[1]. Migraine results in $17 billion of annual health costs in the U.S. alone. In a study[2] conducted with 2,444 people suffering from migraine, two-thirds delayed or avoided taking their prescription medications due to concerns about adverse side effects, and 79% sought products with similar efficacy but fewer side effects.
The FDA market authorization is based on the results of a prospective, randomized, double-blind, placebo-controlled, multi-center pivotal study, where 252 patients from 12 clinics used the non-invasive wearable to treat their migraine attacks.
“This study followed the latest edition of the guidelines from the International Headache Society for controlled trials of acute treatment of migraine attacks in adults,” said Dr. Brian Grosberg, MD, director of the Hartford Healthcare Headache Center in Connecticut, who served as the lead Principal Investigator (PI) of the study.
“The results of the study demonstrate a high efficacy ratio for single as well as multiple attacks, both at two and 48 hours after treatment,” explained Dr. Grosberg, who was a co-author of an article describing the study, released earlier this month in Headache: The Journal of Head and Face Pain published by Wiley Periodicals, Inc. on behalf of the American Headache Society.
Nerivio Migra, a first-in-category product, is placed on the upper arm (not the head or neck) and uses smartphone-controlled electronic pulses to create a Conditioned Pain Modulation (CPM) response. Nerivio Migra® is indicated for acute treatment of migraine with or without aura in adult patients who do not have chronic migraine.
“While the company is preparing to launch the Nerivio Migra® in the United States market later this year at an affordable price, we remain committed to continuing our clinical development, expanding the use of remote electrical neuromodulation therapy for additional indications,” said Alon Ironi, CEO and co-founder of Theranica. “We have identified at least 7 different painful conditions that may be relieved by this non-invasive, drug-free technology after appropriate clinical development.”
“Physicians who treat people with migraine are both patient-centered and science-driven,” said Prof. Stephen Silberstein, director of the Headache Center at the Jefferson University Hospital in Philadelphia, a member of the medical advisory board of Theranica. “Over the last 20 years my colleagues and I have used triptans and ergots for acute migraine treatment. There is a large unmet need for new treatments in this population when these medications are not effective, are contra-indicated, or have non-tolerable side effects. In addition, triptans and most current acute migraine medications, including over-the-counter drugs indicated for migraine, are associated with medication-overuse headache (MOH), which is associated with increased frequency of migraine attacks, and often results in chronic migraine. This new innovative FDA-authorized treatment is an important alternative to help our patients control this debilitating condition.”
[1] Steiner TJ et al. Migraine: the seventh disabler. The Journal of Headache and Pain 2013
[2] Gallagher, Kunkel. Migraine Medication Attributes Important for Patient Compliance: Concerns About Side Effects May Delay Treatment,Headache, 2003
