
Foundation Medicine’s Dr. Gaurav Singal shares how his company’s platform is changing the game in clinical trials and bolstering precision medicine through genomics.
The evolution may be slow going, but it looks like precision medicine is destined to disrupt many aspects of healthcare, and it’s already demonstrating that transformative power in clinical trials—where patient recruitment and retention have long plagued the process, according to Foundation Medicine’s Dr. Gaurav Singal.
“Current clinical trial design and patient identification and recruitment strategies are challenging, especially in a molecular-driven time,” said Singal, vice president of data strategy and product development at the Cambridge, MA-based genomic analysis and diagnostics provider. “Often, patients are narrowly defined and widely distributed geographically. As we worked with a multitude of pharma partners, we began to see this challenge reoccurring; partners were developing medicines for rarer targets and were having significant trouble getting those medicines to patients with those rare targets.”
Satisfying that need to match those more specialized patients and treatments more effectively was the inspiration behind FMI’s Smart Trials technology platform. The company is now using the platform in to rapidly match the right patients to precision trials through genomic testing and the identification of unique tumor biomarkers.
“We have a full suite of comprehensive genomic profiling assays to identify the molecular alterations in a patient’s cancer and match them with relevant, targeted therapies, immunotherapies, and clinical trials. Our molecular information platform aims to improve day-to-day care for patients by serving the needs of clinicians, academic researchers, and drug developers to help advance the science of molecular medicine in cancer,” said Singal.
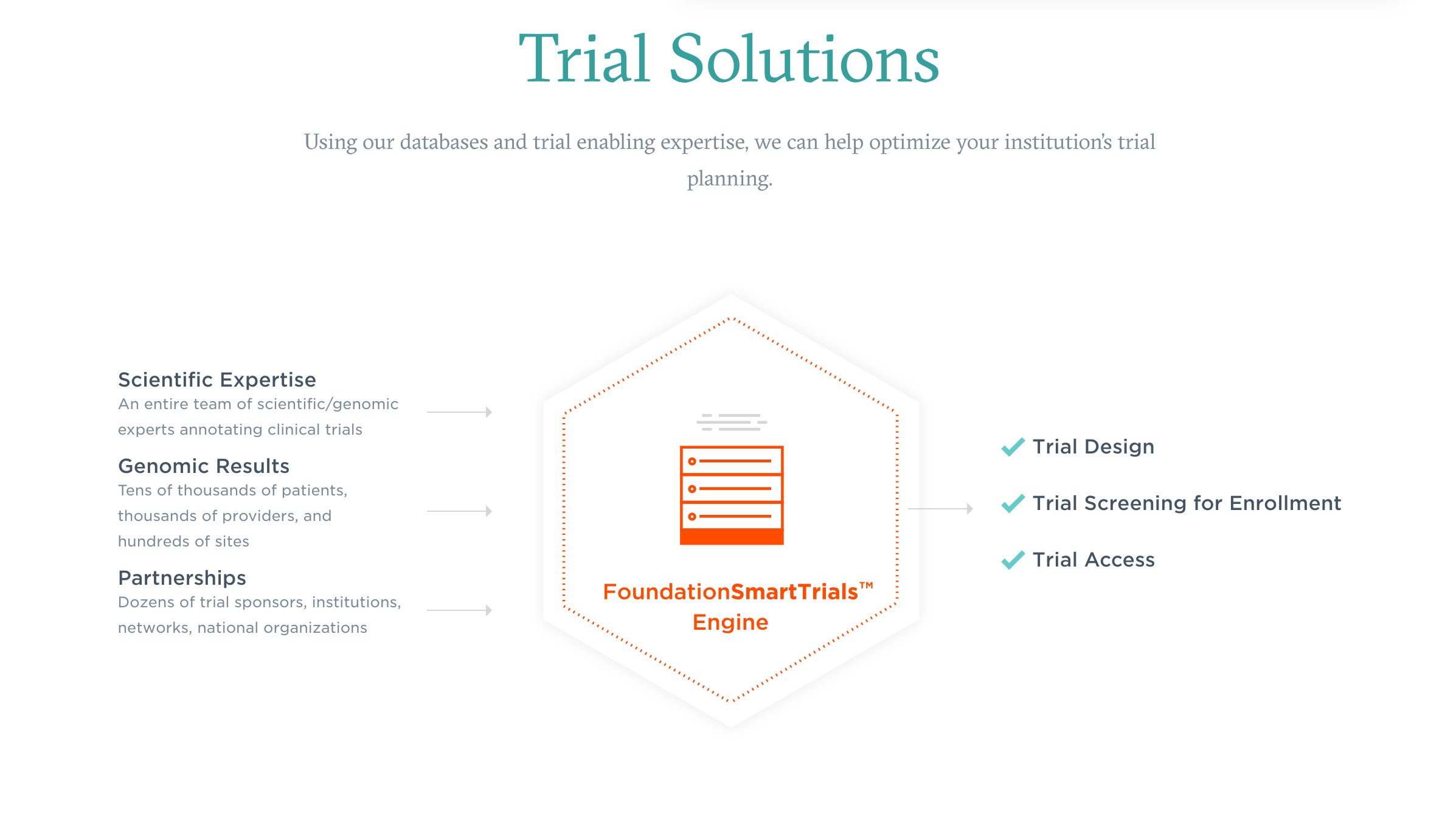
There is no doubt there is reciprocity between the advancements of clinical trials and precision medicine. Precision-focused tools like SmartTrials are uncovering the connections between patients and personalized treatments—and the data from those trials will ultimately be used to further advanced those precision-based tools, care-access techniques, and treatments. It’s with that aim in mind, SmartTrials is already demonstrating its power in several ways within several clinical trials.
Here are five ways this innovative platform is advancing precision medicine research and development and transforming the clinical trial process along the way:
1. Boosting Enrollment: SmartTrials is helping to accelerate patient enrollment in TAPUR, a biomarker-driven clinical trial. Foundation Medicine is leveraging patients’ genomic profiling results to connect them to TAPUR trial sights automatically. Use of SmartTrials in TAPUR has already led to a significant increase in patient enrollment, reporting more than 60 patients within a few months of implementing the technology at one site.
2. Advancing Access and Eligibility: SmartTrials is also supporting enrollment for The National Cancer Institute’s (NCI) “MATCH” precision medicine trial. SmartTrials will automatically notify physicians when their patients’ genomic profiling results make them eligible for a treatment arm within the trial.
3. Improving Drug Development: Research results recently highlighted how SmartTrials helped match non-small cell lung cancer patients to a Phase II trial based on their biomarker. Researchers concluded that these “innovative partnerships, combined with computational matching infrastructure, could improve drug development and therapeutic access.”
4. Demonstrating the Benefit of Genomic Matching: Loxo’s Larotrectinib could be available to patients with the TRK biomarker as early as next year and Merck’s Keytruda was FDA approved recently for patients with the MSI biomarker. Both therapies are evidence that more patients could benefit from joining clinical trials based on their biomarker.
5. Saving Lives: FMI has witnessed firsthand the personal benefits of genomic matching. One example: a prostate cancer patient named David, who’s genomic testing results guided him to a clinical trial that wasn’t even studying prostate cancer at the time. However, because the therapy was targeted to his cancer’s specific makeup, David’s enrollment turned out to be a lifesaver. He is now in complete remission.
Perhaps the most compelling advancements are the ones that seem the most personal: “Stories like David’s support the benefits of precision medicine-based approaches for clinical research and treatment. As the field discovers therapeutic targets or biomarkers, more patients have the potential to receive lifesaving treatment,” Singal concluded.
