What You Should Know:
- The White House Office of Science and Technology and the U.S. Department of Energy teams up with IBM, and others to fight the spread of the coronavirus (COVID-19) using supercomputers.
- The COVID-19 High Performance Computing Consortium pooling supercomputing capacity to help researchers everywhere better understand COVID-19, its treatments, and potential cures.
- These high-performance computing systems allow researchers to run very large numbers of
Read More
Public Health
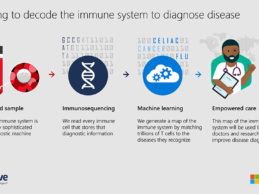
Microsoft, Adaptive, Providence Partner to Decode COVID-19 Immune Response
What You Should Know:
- Microsoft and Adaptive Biotechnologies have expanded their partnership to map the human immune response to COVID-19 and advance our understanding of the disease.
- Data from this study will be made available through an open-access portal to help researchers, public health organizations and industry improve diagnostics, help triage patients based on the immune response to the disease, and inform vaccine discovery for COVID-19.
- Other industry leaders including
Read More
New Website Helps People Find Nearby COVID-19 Testing Centers Nationwide
What You Should Know:
- Chicago-based technology company Evive launched the Evive.Care website, which is helping people find COVID-19 (Coronavirus) testing centers near them.
- The Evive.Care search tool allows people to enter their state and county and then displays a list of nearby COVID-19 testing centers.
- There are currently 240+ testing centers on the website across approximately 200 counties in the U.S.—those numbers are constantly evolving.
Evive, a Chicago,
IL-based
Read More
At-Home COVID-19 Test Kits Will Be Available to U.S. Consumers for $139 on March 23rd
What You Should Know:
- Everlywell announces that an at-home collection kit with telehealth diagnosis for COVID-19 will be available to consumers starting Monday, March 23 for $139.
- The initial offering of 30,000 collection kits will significantly impact total coronavirus testing capacity in the United States; free telehealth consults are included for those with positive results.
- By working with multiple labs to scale infrastructure, the company plans to have testing and diagnosis
Read More
Stanford Alumni Scientists, Physicians Launch StartX Med COVID-19 Task Force
What You Should Know:
- More than 1500 Stanford faculty and alumni announced the launch of StartX Med COVID-19 Task Force featuring 70+ StartX Med Innovators with medical breakthroughs for the prevention, diagnostics, and treatment of coronavirus mobilize efforts to fast-track public health needs during the pandemic.
- The StartX Med COVID-19 Task Force will collaborate on outreach to government agencies, regulatory bodies and healthcare systems in the interest of public health for
Read More
Blue Shield of CA Offers COVID-19 Screener and Emergency Response Assistant
What You Should Know:
- Blue Shield of California offers COVID-19 Screener and Emergency Response Assistant (COVID-19 SERA) to help handle anticipated surge of COVID-19 patients.
- The COVID-19 SERA is developed by GYANT can be customized for each health system's emergency response plan, and it is updated in real-time with latest guidelines from the Centers for Disease Control (CDC) and World Health Organization (WHO).
Blue Shield of California is offering a new digital tool
Read More
Coronavirus Drives Teladoc’s Daily Virtual Medical Visits Up 50%
What You Need to Know:
- In response to the coronavirus (COVID-19) pandemic, Teladoc Health reports that its daily virtual medical visit volume has spiked 50% over the prior week.
- Teladoc Health has provided approximately 100,000 virtual medical visits to patients in the United States in the past week.
Virtual
care provider Teladoc Health
announced that the company is experiencing unprecedented daily visit volume in
the United States as the novel coronavirus (COVID-19) continues
Read More
Vivify Health Launches COVID-19 Screening, Self-Isolation and Monitoring Pathways
What You Need to Know:
- Vivify Health has announced that availability today of new COVID-19 Screening, Self-Isolation and Monitoring Pathways for Vivify+Go mobile solution to providers at non-cost.
- This enables low-risk patients or those with mild symptoms to use their mobile devices to self-screen for COVID-19 by answering a series of questions that follow the current Centers for Disease Control and Prevention (CDC) guidelines.
- The self-screening helps providers scale their
Read More
CTA Releases Virtual Care Guiding Principles, Led by Livongo, Validic, Others
What You Need to Know:
- Consumer Technology Association (CTA) released a new set of industry-led guidelines on virtual care, convening health tech leaders like Doctor on Demand, Livongo, Validic, and BioIntelliSense.
- The virtual care principles offer core guidelines and practices across patient engagement, standards of care, quality, continuity of care, prescribing and privacy and security.
- While virtual care is not a panacea, in the midst of an outbreak, evaluation, triage and
Read More
6 Coronavirus (COVID-19) Considerations for Telehealth Providers
As the novel coronavirus outbreak continues, the federal government and commercial health insurers have taken significant steps to increase Americans’ access to treatment and testing. In the past week, the federal government and private insurers have issued a number of guidance documents expanding coverage and payment requirements in an effort to minimize the spread of the virus.
As with any changes in coverage and reimbursement, healthcare providers offering telehealth services should
Read More